2. Institute of Chemical Materials, China Academy of Engineering Physics, Mianyang 621900, China
Recently, materials with high nitrogen content such as triazole, tetrazole, and tetrazine derivatives have received attention due to their high densities[1-2], high heats of formation[3-4], and high thermal stability[5-6]. The 3-and 6-positions of s-tetrazine have high activity and can be substituted by a soft neutral heteroatom or carbanion nucleophiles, so the interest of s-tetrazine-based compounds has stimulated[7-10]. Now, a novel nitrogen-rich energetic salt of 3-guanidino-6-methoxy-s-tetrazine picrate (MGTZ)(PA) was obtained by reacting 3-methoxy-6-guanidine-s-tetrazine (MGTZ) with picric acid (PA).
The X-ray single crystal data collections for (MGTZ)(PA) were performed on a Rigaku-AFC10/Saturn 724 + CCD diffractometer. The data were collected at 103(2) K in the range 3.06°≤θ≤27.51°. The structure was solved by direct methods using SHELXS-97[11] and refined by means of full-matrix least-squares on F2 with SHELXL-97[12]. Detailed information concerning crystallographic data collection and structure refinement is summarized in Table 1.
| 表 1 Crystallographic data and structure determination details for (MGTZ)(PA) |
Fig. 1 shows the molecular unit of (MGTZ)(PA) with the atom labeling scheme. There is one 3-methoxy-6-guanidine-s-tetrazine cation (MGTZ+) and one picric acid anion (PA-) in the molecular unit of the title compuond. The O(2) atom of PA loses a proton to form PA- anion and MGTZ+ cation is formed by MGTZ accepted the proton due to the N(6) atomic power of the C=N of the MGTZ more stronger than other N atoms in the reaction. So the stable salt (MGTZ)(PA) is generated through electrostatic forces of molecules.
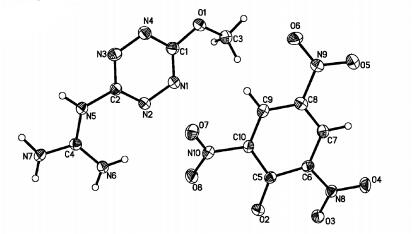
|
图 1 Molecular unit and labeling scheme of (MGTZ)(PA) |
In the MGTZ+ cation, the bond lengths of C—N are from 0.1321(3) nm to 0.1385(3) nm and the average bond length is 0.1341 nm. The value of average C—N bond length is between ordinary C—N single bond(0.1450 nm) and the general C=N double bond(0.1270 nm). Results shows that there is no typical C—N and C=N in the tetrazine ring and connected guanidine, but there is existed effect of conjugated and the effect contributes to the stability of MGTZ+ cation. This effect is mainly produced by a big π bond of the tetrazine ring and a delocalized π-bond from the C—N and C=N of the guanidine.
In the PA- anion, the electron density of the carbon atoms connected with nitro group is decreased due to the electron withdrawing of the nitro group. The bond angles of C(5)—C(6)—C(7), C(5)—C(10)—C(9) and C(7)—C(8)—C(9) are greater than 120°, and the results measured are 123.7(2)°, 124.4(2)° and 121.3(2)°, respectively. However, the electron density of C(5) atom increases because of the oxygen atom of hydroxyl group and benzene ring forming p-π conjugation and the electron cloud excludes two electrons of sp2 hybrid orbitals which makes the bond angle of C(6)—C(5)—C(10) less than 120° and the experiment measured is 112.0(2)°. Meanwhile, the bond length of C(5)—O(2) is 0.1251(3) nm between the normal C—O(0.1430 nm) and C=O(0.1220 nm). Besides, the bond length of C—C tends to averaging and the rang of single bond length is 0.1368(3) nm [C(9)—C(10)] to 0.1451(3) nm [C(5)—C(10)] and the average bond length is 0.1402 nm owing to the conjugated π-bond of benzene ring.
From the packing diagram (Fig. 2) it can be seen that there are extensive intra-molecular and intermolecular hydrogen bonds in the crystal structure of (MGTZ)(PA). The intra-molecular hydrogen bonds are formed between N(6) and N(2) of MGTZ+ cation such as N(6)—H(6B)…N(2) and the N atoms of MGTZ+ cation and the O atoms of PA- anion form the intermolecular hydrogen bonds. A large number of hydrogen bonds make the crystal structure more stable.
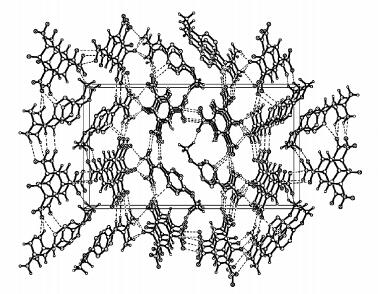
|
图 2 The packing diagram of (MGTZ)(PA) |
Based on the above molecular structure and crystal data, it′s showed that a novel nitrogen-rich energetic salt of (MGTZ)(PA) was synthesized by reacting MGTZ with PA and the crystal was characterized using X-ray single-crystal diffraction. The crystal of the compound belongs to the orthorhombic system, space group P212121 with a=0.48882(11) nm, b=1.3279(3) nm, c=2.3032(5) nm, V=1.4950(6) nm3 and Z=4.
| [1] |
Chavez D E, Hiskey M A, Gilardi R D. 3, 3-Azobis(6-amino-1, 2, 4, 5-tetrazine): a novel high-nitrogen energetic material[J].
Angew Chem Int Ed, 2000, 39: 1791-1793. DOI:10.1002/(SICI)1521-3773(20000515)39:10<1791::AID-ANIE1791>3.0.CO;2-9 |
| [2] |
Kerth J, Löbbecke S. Synthesis and characterization of 3, 3-azobis(6-amino-1, 2, 4, 5-tetrazine) DAAT-a new promising nitrogen-rich compound[J].
Propellants Explos Pyrotech, 2002, 27: 111-118. DOI:10.1002/1521-4087(200206)27:3<111::AID-PREP111>3.0.CO;2-O |
| [3] |
Neutz J, Grosshardt O, Schaufele S, et al. Synthesis, characterization and thermal behaviour of guanidinium-5-aminotetrazolate (GA)-a new nitrogen-rich compound[J].
Propellants Explos Pyrotech, 2003, 28: 181-188. DOI:10.1002/(ISSN)1521-4087 |
| [4] |
Ciezak J A, Trevino S F. The inelastic neutron scattering spectra of a-3-amino-5-nitro-1, 2, 4-2H-triazole: experiment and DFT calculations[J].
Chem Phys Lett, 2005, 403: 329-333. DOI:10.1016/j.cplett.2005.01.033 |
| [5] |
Levchik S V, Balabanovich A I, Ivashkevich O A, et al. The thermal decomposition of aminotetrazoles. Ⅱ. 1-Methyl-5-aminotetrazole and 1, 5-diaminotetrazole[J].
Thermochim Acta, 1993, 225: 53-65. DOI:10.1016/0040-6031(93)85082-K |
| [6] |
Lesnikovich A I, Ivashkevich O A, Levchik S V, et al. Thermal decomposition of aminotetrazoles[J].
Thermochim Acta, 2002, 388: 233-251. DOI:10.1016/S0040-6031(02)00027-8 |
| [7] |
Audebert P, Sadki S, Miomandre F, et al. New functionalized tetrazines: synthesis, electrochemical and spectroscopic properties[J].
New J Chem, 2004, 28: 387-392. DOI:10.1039/B310737J |
| [8] |
Hu W X, Rao G W, Sun Y Q. Synthesis and antitumor activity of s-tetrazine derivatives[J].
Bioorganic & Medicinal Chemistry Letters, 2004, 14: 1177-1181. |
| [9] |
Saikia A, Sivabalan R, Polke B G, et al. Synthesis and characterization of 3, 6-bis(1H-1, 2, 3, 4-tetrazol-5-ylamino)-1, 2, 4, 5-tetrazine (BTATz): Novel high-nitrogen content insensitive high energy material[J].
Journal of Hazardous Materials, 2009, 170: 306-313. DOI:10.1016/j.jhazmat.2009.04.095 |
| [10] |
Shawali A S, Elsheikh S M. Annelated[1, 2, 4, 5]tetrazines[J].
J Heterocycl Chem, 2001, 38: 541-559. DOI:10.1002/jhet.v38:3 |
| [11] |
Sheldrick G M. SHELXS-97, Program for the Solution of Crystal Structure[M]. University of Göttingen, Germany, 1997.
|
| [12] |
Sheldrick G M. SHELXL-97, Program for the Refining of Crystal Structure[M]. University of Göttingen, Germany, 1997.
|

A novel nitrogen-rich energetic salt of 3-guanidino-6-methoxy-s-tetrazine picrate (MGTZ)(PA) was synthesized by reacting 3-methoxy-6-guanidine-s-tetrazine (MGTZ) with picric acid (PA). The crystal structure was obtained and determined by single-crystal X-ray diffraction.




