Gas-generating propellants[1], which could produce large amounts of cool and clean gases in a certain given time, were widely used in missiles and rocket motors. Oxidizers, such as ammonium nitrate, ammonium perchlorate, dihydroxyglyoxime, nitramines(HMX or RDX) etc were introduced into propellant compositions successively in order to design high performance gas-generating propellant. Due to nitramine-based composite modified double base(CMDB) propellant possesses high specific impulse(Isp) in addition to non-smoke, non-corrosive and non-toxic exhaustive products[2], CMDB propellant is the preferred choice for gas-generating propellant. Guanidinium azotetrazolate(GZT), whose nitrogen content is 78.84%, formation enthalpy is 1448.16 kJ·kg-1, decomposition peak temperature is 240 ℃ and gas output is 1103.3 L·kg-1[3], was used in propellants to modify the combustion behaviors and reduce the combustion temperature[4-6]. In view of its great abilities to reduce the combustion temperature and increase the gas output, some patents on gas generator formulations containing GZT were acclaimed in civil devices[7-8], otherwise, RDX-CMDB propellant gas generator containing GZT with high gas output and low combustion temperature used in military arms has not been reported yet. In this paper, characteristics of RDX-CMDB propellants with RDX replaced stepwise by GZT at the rate of 3% were studied in order to design high performance solid propellant gas generator used in missiles or rockets.
2 Experiments and Methods 2.1 MaterialsGuanidinium azotetrazolate(GZT), whose purity is over 99.9%, was synthesized in the laboratory on the basis of the reported procedures[9]. Cyclotrimethylenetrinitroamine(RDX), nitrocellulose(NC, 12.0 % N), nitroglycerin(NG), catalysts(lead and copper organic salts) and other auxiliary additives were procured.
2.2 ProcedureThe typical RDX-CMDB propellant composed of 25% NC, 31% NG, 40% RDX, and 4% other auxiliary additives was used as the control formulation in this experiment. Propellants with RDX replaced stepwise by GZT at the rate of 3% were prepared by the slurry-cast technique, including slurry mixing, casting, degasing by vacuum pump and curing. Composite catalysts composed of copper and lead salts were added in order to modify the combustion behaviors of propellants.
2.3 MethodologyThermodynamic performance calculations were carried out with REAL software[10] at a standard expansion ratio of 70:1(the pressure in the combustor is 7 MPa). Standard theoretical specific impulse(Isp), combustion temperature(Tc), explosion heat(Q) and molar output of gases(n) were calculated.
Explosion heat at constant volume(Qv) was measured by a Model ZDHW-2 temperature-constant automatic calorimeter(China) with sample mass of 5.0 g and reference sample of standard double-based gun propellant in vacuum. The detailed operations can refer to the method GJB 772A-1997.701.1.
The burning behaviors were determined by measurements on strands of 100 mm length and 5 mm×5 mm square cross section which were covered with polyvinyl alcohol. Burning times and rates were determined for 80 mm measuring distance by melting down of 10 mm diameter lead wires drawn through the strands. The detailed operations can refer to the method GJB 770B-2005.706.1.Vacuum stability test was performed as GJB 772A-1997.501.1 in a Model YC-1 instrument(China) with sample mass of 5.00 g at 90 ℃ for 48 h. 5 s explosion point was determined according to GJB 772A-1997.606.1 in a wood′s metal bath with 0.03 g sample. Methyl Violet Test, according to GJB 770A-1997.503.3, time for changing the color of methyl violet paper to salmon pink was recorded using a stop watch when the composition was subjected to methyl violet(MV) test at 120 ℃.
TG measurements were carried out on a TG instrument(model 2950, TA, USA). The test conditions were as follows: sample mass is about 0.8 mg; heating rate is 10 ℃·min-1; 0.1 MPa, dynamic nitrogen atmosphere with a flowing rate of 50 mL·min-1.
DSC measurements were carried out on a TA instrument(model DSC910S, TA, USA). The test conditions were as follows: samples mass is about 0.7 mg; heating rate is 10 ℃·min-1; 0.1 MPa, dynamic nitrogen atmosphere with a flowing rate of 50 mL·min-1. The mass ratio of binary mixed system is 1:1.
The solid residual mass was measured by lighting 0.4 g sample in a pot under nitrogen atmosphere with the pressure 3 MPa.
Configurations and compositions of quenched surfaces obtained by fast-cooling method using coppery platform under 3 MPa were studied using scanning electron microscope(SEM) and LINK ISIS energy spectrum.
3 Results and Discussions 3.1 Thermal dynamic propertiesCharacteristics in terms of the standard theoretical specific impulse(Isp), combustion temperature(Tc), explosion heat(Q) and gas output were calculated theoretically and solid residual weight was measured experimentally. The results were listed in Table 1.
| Tab.1 Results of theoretically calculated thermal dynamic properties of propellants containing GZT |
As showed in Table 1, the combustion temperature, specific impulse, explosion heat and solid residue decreased gradually with replacement of RDX by GZT, while the gas volume increased, which justly meet the requirements of propellants used as gas generators. It′s also observed from Tab. 1 that the experimentally determined values of explosion heat were in good agreement with that of theoretic calculations.
The low Tc of GZT-RDX based CMDB propellant is attributed to the higher nitrogen content(78%)[11] of GZT as compared to that of RDX(37%) and the lower adiabatic combustion temperature of GZT(687 K) as against that of RDX(3284 K)[12]. Although the formation heat of GZT(1448.16 kJ·kg-1) is much more than that of RDX(276.78 kJ·kg-1), the specific impulses decreased with the replacement of RDX by GZT. The decrease of specific impulses may be attributed to the significant decrease in the oxygen balance of GZT(-78%) as compared to that of RDX(-21.6%) and lower explosion heat of GZT as compared to that of RDX. The low temperature and large amount of nitrogen gases contributed greatly to the formation of less erosive gas products and low mean molecular weight products which resulted in the increase of gas volume.
3.2 Combustion properties 3.2.1 Combustion behaviors of propellants without the catalystCombustion behaviors including burning rates and pressure exponents of propellants without the catalyst under 1 MPa, 3 MPa, 5 MPa, 7 MPa and 10 MPa were studied. Results of burning rates and pressure exponents of propellants and element analysis of quenched surfaces were showed in Fig. 1 and Tab. 2, respectively.
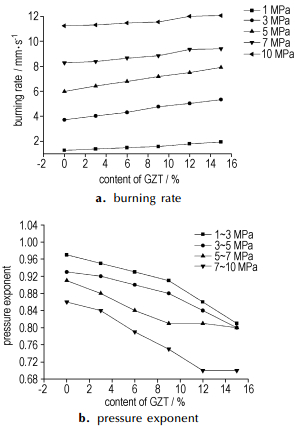
|
Fig.1 Trend of burning rate(a) and pressure exponent(b) of propellants without the catalyst |
| Tab.2 Element analysis results of quenched surfaces of propellants without the catalyst |
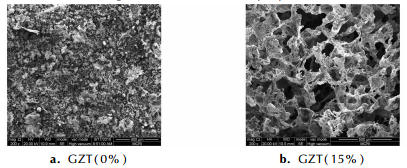
As showed in Fig 1, under a certain pressure, the burning rate increased and pressure exponent decreased with replacement of RDX by GZT. The phenomenon can be explained through the following three aspects. Firstly, as showed in Fig. 2, the decomposition peak temperature GZT/RDX system is 217.79 ℃ which is 21.71 ℃ and 44.38 ℃ lower than the individual decomposition peak temperature of RDX(239.50 ℃) and GZT(262.17 ℃), respectively, which indicated that GZT(RDX) could accelerate the decomposition of RDX (GZT). Secondly, as shown in Fig. 2, no melting process was observed before the decomposition of GZT in the DSC curve of GZT, which indicated that GZT decomposed directly at solid state. Due to the fact that the melt layer phenomenon that occurred during combustion of RDX had been reported to be responsible for the lower burning rate and hardly modified high pressure exponent for the RDX-CMDB propellants[13]. Last but not the least, the carbons on the surface produced by the combustion of high negative oxygen balance GZT(-78.8%) can broke up the melt layer too and hence improve the heat and mass transfer. This point can be testified through the research results of quenched surfaces of propellants as shown in Tab. 2 and Fig. 3. From Tab. 2, we can see when GZT was added into propellant, more carbons were formed. From Fig. 3(a), we can see an integrate melted layer formed on the surface which was the main heat and mass transfer control region. When GZT was added, more carbons were formed on the surface and the integrate melt layer disappeared as shown in Fig. 3(b).
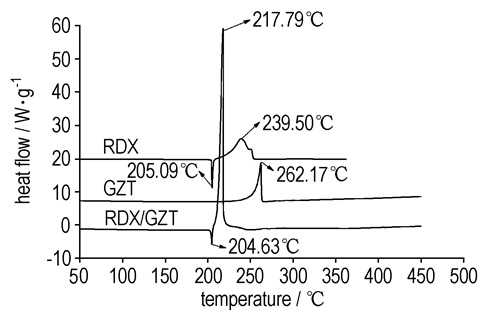
|
Fig.2 DSC curves of system GZT, RDX and GZT/RDX(mass ratio is 1:1) |

|
Fig.3 SEM photographs of quenched surface of propellant containing 0% GZT(a) and 15% GZT(b) without the catalyst |
The combustion behaviors including burning rates and pressure exponents of propellants with the catalyst under 1 MPa, 3 MPa, 5 MPa, 7 MPa and 10 MPa were studied. Results of burning rates and pressure exponents of propellants and element analysis of quenched surfaces were showed in Fig. 4 and Tab. 3, respectively.

|
Fig.4 Trend of burning rate(a) and pressure exponent(b) of propellants with catalyst |
| Tab.3 Element analysis results of quenched surfaces of propellants with the catalyst |
Compared to combustion behaviors of propellants without the catalyst, the burning rate of propellants with the catalyst were almost twice times than that of propellants without the catalyst at the same level of GZT content, which indicated that catalyst has more effects on combustion behaviors of RDX-CMDB propellants than GZT does. The difference is that the burning rate increases and pressure exponent decreases with the increase in GZT content for the propellants without the catalyst, while the burning rate decreases and pressure exponent increases with the increase in GZT content for the propellants with the catalyst, which can be explained through the following two aspects.
Firstly, as showed in Fig 5, the decomposition peak temperature of GZT, NC+NG and RDX were brought forward 0 ℃, 24.9 ℃ and 17.3 ℃ when GZT, NC+NG or RDX mixed with catalysts, respectively, which indicated that the catalyst had great influences on decomposition of RDX or NC+NG but no influence on decomposition of GZT. This phenomenon also indicated that the catalysts modified the combustion behaviors of RDX-CMDB propellants through accelerating the decomposition of RDX or nitro ester, which is consistent with classic catalysis theories brought forward by Lengell G[14], Singh H[15] and Preckel R.F[16] respectively. They thought that lead salts as catalyst can modify the combustion behaviors through the catalysis on the oxidation-reduction reactions between CHO, C and NO, NO2, or effect on the break of NO2 groups from the molecular. Neither NO2 group existed in GZT molecular nor NO or NO2 gas were produced in the decomposition process of GZT[6, 9], and the main decomposition product N2 doesn′t react with lead or lead oxide, so the heat produced by the oxidation-reduction reaction between NO or NO2 and C or CHO per unit mass propellant in unit time reduced and the burning rates decreased with replacement of RDX by GZT.
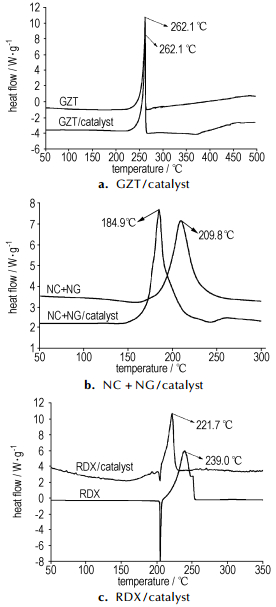
|
Fig.5 DSC curves of system GZT/catalyst(a), NC+NG/catalyst(b) and RDX/catalyst(c) |
Secondly, the catalyst[17] could improve combustion behaviors because more carbons which were considered as the catalysis carriers were formed when lead and copper catalysts were added into RDX-CMDB propellants, which can be testified from the carbon element analysis results and SEM photo(in Fig. 3(a) and Fig. 6) of propellants′ quenched surfaces. When GZT and the catalyst were added simultaneously into propellants, superfluous carbon formed as showed in Fig. 6(b) owning to incompletely combustion of GZT. Those redundant carbons formed an adiabatic layer[18] and block the heat transfer from gas phase to condense phase, so the burning rate decreases with the replacement of RDX by GZT. The influences of carbon on behaviors of RDX-CMDB propellants indicates that certain amount of carbon products can increase the burning rate, and redundant carbon products can form an adiabatic layer and decrease the burning rate of RDX-CMDB propellants.
|
Fig.6 SEM photographs of quenched surfaces of propellant containing 0% GZT(a) or 15% GZT(b) with the catalyst |
The thermal stabilities of propellants with catalysts were studied by MV test, 5 s explosion point test and Vacuum stability test, respectively. The results were listed in Tab. 4.
| Tab.4 Thermal stabilities of propellants |
As showed in Tab. 4, 5 s explosion point decreased with addition of GZT, which indicated that propellants containing GZT inclined to deflagrate easily at high temperature above 200 ℃ with increase of GZT content. While, the methyl violet paper changing time changed lightly and rulelessly, and gas volume in vacuum stability tests decreased which indicated that thermal stability is acceptable below 100 ℃.
3.4 Thermal behaviors of propellantsThe Thermal behaviors of propellants without the catalyst were studied through TG-DTG experiment. TG-DTG curves of propellants containing no GZT or 15% GZT were showed in Fig. 7.

|
Fig.7 TG curves of propellants containing 0% GZT(a) and 15% GZT(b) without the catalyst |
The TG-DTG curves of propellant containing 0% GZT consisted of two mass loss stages with mass loss 23.83% and 62.39%, respectively, corresponding to volatilization of NG and decomposition of NC and RDX respectively. TG curve of propellant containing 15% GZT has three mass loss stages with mass loss 24.8%, 42.47% and 7.45%, respectively. The first mass loss corresponds to volatilization of NG. The second mass loss corresponds to decomposition of NG, NC, RDX and partial GZT because of interactions between RDX and GZT as described in 3.2.1. The third mass loss corresponds to the decomposition of GZT with the peak temperature 271.9 ℃ which is brought back compared to the DSC peak temperature 262.1 ℃, which may be due to the interactions between GZT and components of propellant.
4 ConclusionsThe abilities of GZT to decrease combustion temperature, solid residue mass and simultaneously increase the gas output of RDX-CMDB propellants make it possible to apply in high performance gas generators. GZT increases the burning rate and decreases the pressure exponent of RDX-CMDB propellants without the catalyst(copper and lead salts) and has the opposite effects on combustion behaviors of propellants with the catalyst. GZT makes RDX-CMDB propellants deflagrate and detonate more easily at high temperature, but has no obvious influence on thermal stability of propellants at low temperature below 100 ℃. TG experiments indicate that partial GZT decomposes independently.
| [1] |
Helmy A M, Selph T M, Hollister. GAP propellant for gas generator application[R]. AIAA-87-1725, 1987.
|
| [2] |
Raman K V, Singh H. Ballistic modification of RDX-CMDB propellants[J]. Propellant, Explosive and Pyrotechnic, 1988, 13: 149-151. DOI:10.1002/(ISSN)1521-4087 |
| [3] |
Menke K, Gunser G, Schnoering M, et al. Burning rate enhancement of AN-propellants by TAGN and GZT[C]//34th International Annual Conference of ICT, Karlsruhe. 22, 2003.
|
| [4] |
Menke K, Gunser G, Bohnlein J, et al. Combustion modification of AN propellants with energetic binders by TAGN and GZT[C]//9-IWCP Workshop on Combustion and Propulsion, Lerici, Italy, 2003.
|
| [5] |
Menke K, Born M, Kempa P B. AN/PolyGLYN propellants-minimum smoke propellants with reduced sensitivity[C]//37th International Annual Conference of ICT, Karlsruhe. 14, 2006.
|
| [6] |
Sivabalan R, Talawar M B, Senthilkumar N, et al. Studies on azotetrazolate based high nitrogen content high energy materials potential additives for rocket propellants[J]. J Therm Anal Cal, 2004, 78: 781-792. |
| [7] |
Bucerius K M, Eisenreich N, Schmid H, et al. Gas generating mixture containing copper diammine dinitrate: US5663524[P]. 1997.
|
| [8] |
Khandhadia P S, Burns S P. Thermally stable nonazide automotive air-bag propellants: US 6306232[P]. 2001.
|
| [9] |
Hammerl A, Hiskey M A, Holl G, et al. Azidoformamidinium and guanidinium 5, 5'-azotetrazolate salts[J]. Chem Mater, 2005, 17(14): 3784-3793. DOI:10.1021/cm050684f |
| [10] |
Belov G V. Termodinamichesky analiz produktov sgoraniia pri vysokih davelniiah[C]//Vestnik MGTU(Russia), 1993, 2: 43-46.
|
| [11] |
Hiskey M, Goldman, Stine. High-nitrogen energetic materials derived from azotetrazolate[J]. J Energetic Materials, 1998, 16(2-3): 119-127. DOI:10.1080/07370659808217508 |
| [12] |
Ren Wu-zheng. Theoretic and Practice of Explosives and Pyrotechnics[M]. Beijing: China North Chemical Industries Corp. 2001: 259.
|
| [13] |
LIU Zi-ru, LIU Yan, FAN Xi-ping, et al. Thermal decomposition of RDX and HMX part Ⅰ: Characteristic values of thermal analysis[J]. Chinese Journal of Explosives and Propellants, 2004, 27(2): 63-66. |
| [14] |
Lengell G, Izot A, Duteroue J, et al. Steady state burning of homogeneous propellants[M]//Kuo Kenneth K, Summerfield Martin. Fundamental of Solid Propellants Combustion. Washington D C: AIAA, 1984, Vol. 90, Chapter 7.
|
| [15] |
Singh H, Rao K R. Mechanism of combustion of catalyzed double base propellants[J]. Combustion and Flame, 1988, 71: 205. DOI:10.1016/0010-2180(88)90008-9 |
| [16] |
Preckel R F. Plateau ballistics in nitrocellulose propellants[J]. AIAA Journal, 1965, 3(2): 346-347. DOI:10.2514/3.2852 |
| [17] |
WANG Han, ZHAO Feng-qi, LI Shang-wen, et al. Function of carbon materials used in solid propellants and their action mechanism[J]. Chinese Journal of Explosives and Propellants, 2006, 29(4): 32-35. |
| [18] |
WANG Jiang-ning. Studies on combustion disciplines of DB and CMDB propellants with catalysts[D]. Beijing: Beijing Institute of Technology, 2004.
|

Characteristics of RDX-CMDB propellants containing guanidinium azotetrazolate (GZT) were investigated and combustion behaviors of RDX-CMDB propellants containing GZT with or without copper and lead organic salts were explained basing on analysis of the thermal interactions between components of propellants and GZT and studying the quenched surfaces of propellants.



