In the last few decades, pentazole derivatives have received a great deal of attention as they are important intermediates in the synthesis of all-nitrogen compounds[1-2]. In order to continue the search for novel energetic pentazole derivatives, 4, 5-dicyanoimidazol-2-yl-pentazole (DCIP) was designed and synthesized from 2-amino-4, 5-dicyanoimidazole by introducing 4, 5-dicyanoimidazolyl group to the pentazole ring. The structure of the pentazole compound was characterized by 15N NMR spectroscopy.
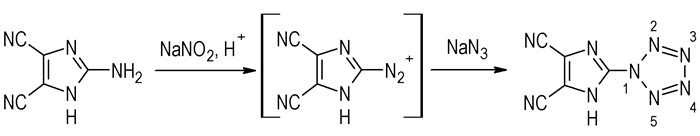
The synthesis of DCIP: 2-amino-4, 5-dicyanoimidazole (1.291 g) was dissolved in a solution of mineral acid and water, and a solution of sodium nitrite (0.76 g) in 2 mL of water was added dropwise at 0 ℃. The above mixture was stirred for 30 min, and cooled to -40 ℃, then a solution of sodium azide (0.71 g) in 10 mL of 50% aqueous methanol was added dropwise. After continuous stirring for 1 h at -40 ℃, the mixture was filtrated and dried at -40 ℃ to give yellow powder (Scheme 1). When sodium nitrite or sodium azide was replaced by 15N labeled sodium nitrite or 15N labeled sodium azide in the above synthesis, sample Ⅰ and sample Ⅱ was obtained, respectively. The 15N NMR of DCIP was determined using deuterated methanol as solvent and nitromethane as external standard.
|
Scheme1 Synthetic route of DCIP |
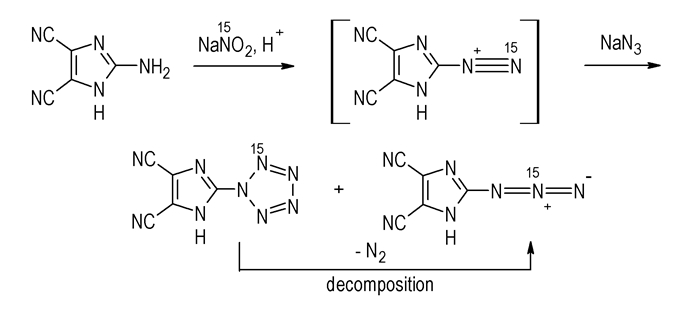
15N NMR results show for sample Ⅰ that the signals of δ -23.57 and δ -143.97 are obtained at -40 ℃, and the signal of δ -23.57 is disappeared when the sample is heated to 20 ℃. It may conclude that sample Ⅰ consists of the N2/5 15N labeled pentazole and Nβ 15N labeled 2-azido-4, 5-dicyanoimidazole (ADCI)[3] (Scheme 2), and δ -23.57 and δ -143.97 can be assigned to N2/5 of DCIP and Nβ of ADCI, respectively.
|
Scheme2 Mechanism of the reaction using 15N labeled NaNO2 |
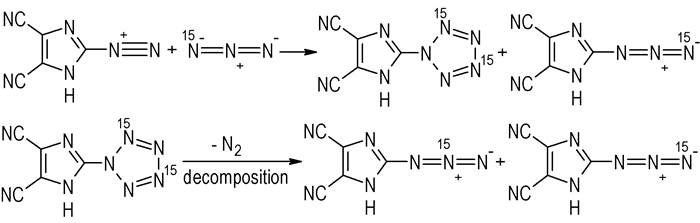
Similarly, for sample Ⅱ, the signals of δ 7.39, δ -23.57 (N2/5), δ -139.15 and δ -143.97 (Nβ) are detected at -40 ℃, and two signals of δ 7.39 and δ -23.57 (N2/5) are disappeared when the sample is heated to 20 ℃. It indicates that sample Ⅱ consists of the N2/5 and N3/4 15N labeled pentazole and Nγ 15N labeled ADCI, Nβ 15N labeled ADCI was formed when the decomposition of pentazole occurred[3] (Scheme 3), and δ 7.39 and δ -139.15 can be assigned to N3/4 of DCIP and Nγ of ADCI, respectively. In addition, the decomposition of DCIP in the synthesis and 15N NMR analyses was confirmed by the signal of Nβ of ADCI detected at -40 ℃.
|
Scheme3 Mechanism of the reaction using 15N labeled NaN3 |
In summary, it is found that the 15N NMR chemical shifts of DCIP are -23.57 (N2/5) and 7.39 (N3/4), and are agreement with the 15N NMR chemical shifts of p-dimethylaminophenylpentazole (-27.1 (N2/5) and 4.9 (N3/4)) reported [4].
| [1] |
Fan S, Wilson K J, Rodney J B. On the stability of N5+N5-[J]. J Phys Chem, A2002, 106: 4639-4644. DOI:10.1021/jp015564j |
| [2] |
Cui J, Zhang Y, Zhao F, et al. HB(N5)3M(M=Li, Na, K and Rb): A new kind of pentazolids as HEDMs[J]. Progress in Natural Science, 2009, 19: 41-45. DOI:10.1016/j.pnsc.2008.06.010 |
| [3] |
Butler R N, Fox A, Collier S, et al. Pentazole chemistry: the mechanism of the reaction of aryldiazonium chlorides with azide ion at -80 ℃: concerted versus stepwise formation of arylpentazoles, detection of a pentazene intermediate, a combined 1H and 15N NMR experimental and ab initio theoretical study[J]. J Chem Soc, Perkin Trans, 2, 1998, 2243-2247. |
| [4] |
Müller R, Wallis J D, Philipsborn W. Philipsborn W. Direct structural proof for the pentazole ring system in solution by 15N-NMR spectroscopy[J]. Angew Chem Int Ed Engl, 1985, 24(6): 513-515. |
4,5-Dicyanoimidazol-2-yl-pentazole is a novel energetic pentazole compound, and its synthesis was achieved using 2-amino-4,5-dicyanoimidazole as starting material. The structural proof for the pentazole ring system by 15N-NMR spectroscopy of 15N labeled samples was presented. Meanwhile, the decomposition of the title compound was confirmed.



