Trifluoroacetic acid (TFA) is corrosive and toxic, which is hard to decompose by chemical reaction and micro-biological degradation[1-2]. However, TFA is largely used for the nitrogen oxidation reaction to obtain 2, 6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105). Common industrial separation methods such as distillation can not be utilized for recovery of TFA because of a maximum boiling azeotrope (20.6 wt % water, boiling at 105.5 ℃ [3]) formed by TFA and water. Till now, there is few report about the separation method of TFA-H2O. Deng[4] et al. studied the recovery of TFA liquid in the synthesis of LLM-105 explosive, the operation processes included neutralization using strong base, concentration, acidation and distillation. Mahajan[5] et al. investigated the feasibility of using reactive distillation to recover TFA through esterification with 2-propanol, but this method was aimed to dilute aqueous solution, which is unsuitable for concentrated aqueous solution.
Considering above, we explored a new broken azeotropic technique to recover TFA by adding solvent A, and then employ atmospheric distillation to recove TFA for synthesis of LLM-105. The process diagram is shown in Scheme 1.
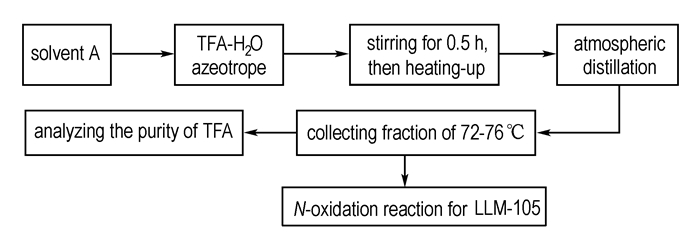
|
Scheme1 The flow process diagram of recovery |
(1) Separation of TFA-H2O azeotrope
A is a strong hydrophilic solvent, and does not react with TFA. At room temperature, 372.2 g solution containing TFA (70 wt %) was added to the three-necked bottle, then solvent A was added slowly. After stired for 0.5 h, the mixture was heated to reflux, and distilled at atmospheric condition.The fraction was collected at 72-76 ℃, and 248.0 g TFA was obtained with a yield of 88%. The purity of TFA was 93% by GC-MS quantitative analysis (see in Figure 1).
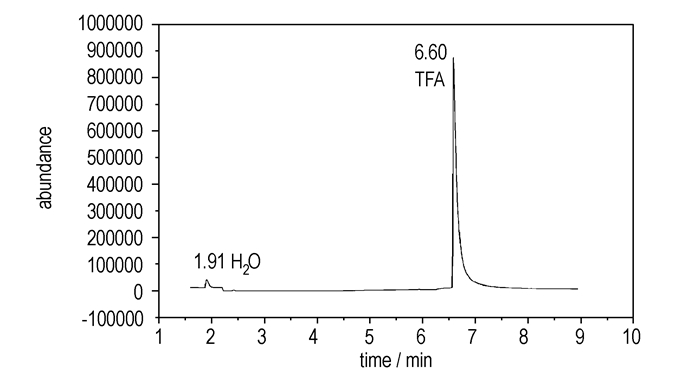
|
Fig.1 The purity of recovery TFA |
(2) N-oxidation for LLM-105 with fresh/recovered TFA
5.0 g 2, 6-diamino-3, 5-dinitropyrazine, 50 mL TFA were added to the three-necked bottle, 15 g 50% H2O2 was dropwised to the mixture in ice bath. It was stirred for 6 h at 25-30 ℃, and the precipitation was filtrated, washed with water, dried at 60 ℃ in vacuum. The experimental results were listed in Table 1, which indicating that the purity of LLM-105 using recovered TFA can be paralleled to that of fresh TFA.
| Tab.1 Comparison of experimental results between fresh and recovered TFA |
A new recovered technique of TFA in the synthesis of LLM-105 explosive was obtained. The yield and purity of recovered TFA were 88% and 93%. The recovered TFA was used in the synthesis of LLM-105 with good yield and purity.
| [1] |
Richey D G, Drisco C T, Likens G E. Soil retention of t rifluoroacetate[J]. Environmental science & technology, 1997, 13(6): 17232-1727. |
| [2] |
Wall Ington T J, Schneider W F, Worsnop D R, et al. The environmental impact of CFC replacement s 2HFCs and HCFCs[J]. Environ Sci Technol, 1994, 28: 320A2326A. |
| [3] |
CHENG Neng-lin. Manual of solvent(second edition)[M]. Beijing: Chemical Industry Publishing Company, 1994, 882.
|
| [4] |
DENG Ming-zhe, YE Zhi-hu, Su Hai-peng, et al. Recovery of TFA in the synthesis of LLM-105 explosive[J]. Chinese Journal of Explosive & Propellants, 2009, 32(4): 50-52. |
| [5] |
Mahajan Y S, Shah M A, Kamath R S. Recovery of trifluoroacetic acid from dilute aqueous solutions by reactive distillation[J]. Separation and Purification Technology, 2008, 59, 58-66. |
A new recycling technique of trifluoroacetic acid in the synthesis of LLM-105 explosive was obtained, and the recovered TFA was used in the synthesis of LLM-105 with good yield and purity.




