Tetrazole-based nitrogen-rich compounds are energetic materials due to their high enthalpy of formation, high density and easy to achieve balance of oxygen[1-4]. 5-nitraminotetrazolate (5-NATZ) has been proved to possess excellent energetic properties among the simple tetrazole compounds[5-6] and its metal complexes were thought to be promising energetic materials[7-8]. In this paper, a novel nitrogen-rich energetic compound of Zn(5-NATZ)2(H2O)4 was synthesized and determined by X-ray single crystal diffraction technology.
Zn(5-NATZ)2(H2O)4 was synthesized by reacting 5-NATZ with Zn(NO3)2·6H2O at 30 ℃ for 3 h and crystallized by slow evaporation method. The X-ray single crystal data collection for Zn(5-NATZ)2(H2O)4 were performed on a Rigaku AFC-10/Saturn 724+CCD diffractometer with graphite monochromated Mo Kα radiation (λ= 0.071073 nm). The datas were collected at 103(2) K in the range of 3.0°≤θ≤27.5°. A semi-empirical absorption correction was made using SADABS software. The structure was solved using the direct methods using SHELXS-97[9], refined using full-matrix least-squares on F2 with SHELXL-97[10]. Detailed information concerning crystallographic data collection and structure refinement are summarized in Table 1.
| Tab.1 Crystal data and structure refinement for the title compound |
Fig. 1 shows the molecular unit of Zn(5-NATZ)2(H2O)4. The ZnⅡ ion with sp3d2 hybridization, contributes six empty orbits to accommodate the lone pair electrons from ligands and to coordinate with two nitrogen atoms from two 5-NATZ ions and four oxygen atoms from four water molecules. The bond angle of the nitrogen atoms N(1), N(1A) from two 5-NATZ ligands and the ZnⅡ ion is 180.00° (N(1)—Zn(1)—N(1A) =180.00°); the bond angle of four water groups and the ZnⅡ ion are 180.0°, 87.53° and 92.47°, respectively (O(3)—Zn(1)—O(3A) = 180.0°, O(3)—Zn(1)—O(4)=87.53°, O(3)—Zn(1)—O(4A)=92.47°), the bond angles of nitrogen atoms N(1)、N(1A)from two 5-NAZT groups, O(3) from four water groups, and the ZnⅡ ion are 90.07° and 89.93°, respectively (O(3)—Zn(1)—N(1)=90.07°, O(3)—Zn(1)—N(1A)=89.93°). At the same time, the Zn-O(terminal water) distances (range from 2.0431 to 2.2409 Å) are similar to the bond lengths (both are 2.1033 Å) of Zn-N(terminal 5-NATZ).These results indicate that the ZnⅡ ion exhibits a slightly distorted octahedral configuration.
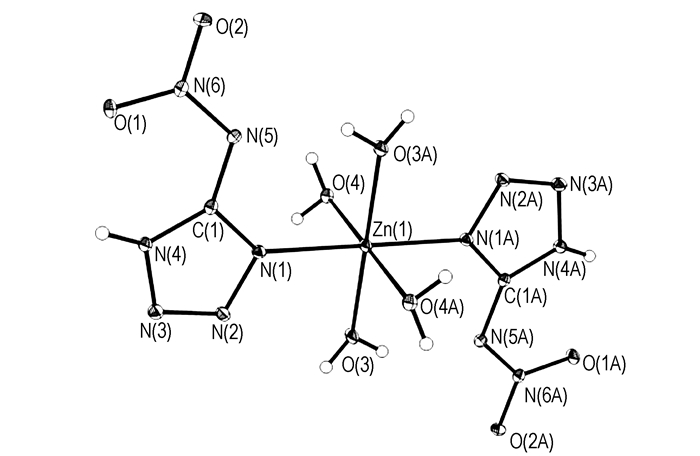
|
Fig.1 Molecular unit of 5-Zn(NATZ)2(H2O)4 |
In the Zn(5-NATZ)2(H2O)4 molecular structure, the 5-NATZ form the plane A, -2.917x+5.984y+2.977z=2.9493, and the deviation is 0.0240. Since the torsion angles of Zn(1)—N(1)—C(1)—N(4)and Zn(1)—N(1)—N(2)—N(3)are -178.31° and 178.40°, respectively. Zn(1) and NATZ almost in the same plane. The plane B formed from O(3), Zn(1), N(1), C(1), N(4), N(3) and N(2) is -2.534x+5.962y+2.935z=3.1051, and the deviation is 0.0513. The angle between A and B is 3.7°. O(4), Zn(1) and N(1) formed the plane C, 6.083x-0.820y-0.770z=2.2467, and the deviation is 0. The angles of the plane C from A and B are 95.8° and 92.1°, respectively, which indicates that the plane A and B are perpendicular to the plane C. The whole molecule is central symmetrical.
The O—H…N weak hydrogen bonds between 5-NATZ groups and water ligands were observed in [Zn(5-NATZ)2(H2O)4]n molecules. It can be seen from the packing diagram (Fig. 2) that all intermolecular hydrogen bonds extend the structure into a 3D supramolecular structure and make an important contribution to enhance the thermal stability of the complex.
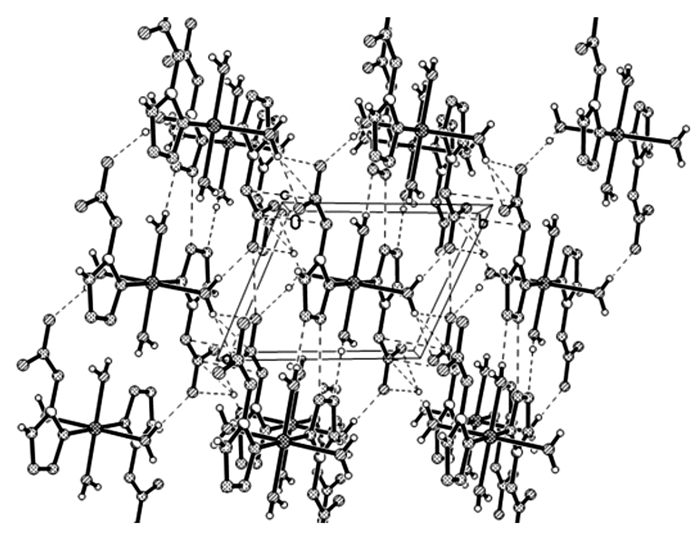
|
Fig.2 Packing diagram of Zn(5-NATZ)2(H2O)4 |
Above all, the novel nitrogen-rich energetic compound Zn(5-NATZ)2(H2O)4 was synthesized from the corresponding 5-NATZ and Zn(NO3)2·6H2O, which was characterized using X-ray single crystal diffraction. The crystal data indicate that the compound belongs to triclinic, space group P-1. Its crystal structure show that the ZnⅡ ion exhibits a slightly distorted octahedral configuration and the whole molecule is central symmetrical. Its intermolecular hydrogen bonds make a vital contribution to the stable 3D architecture.
| [1] |
Huynh M H V, Hiskey M A, Chavez D E, et al. Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen C-N compound[J]. J Am Chem Soc, 2005, 127(36): 12537-12543. DOI:10.1021/ja0509735 |
| [2] |
Neutz J G O, Schaufele S, et al. Synthesis, characterization and thermal behaviour of guanidinium-5-aminotetrazolate(GA)-a new nitrogen-rich compound[J]. Propellants Expls Pyrotech, 2003, 28(4): 181-188. DOI:10.1002/(ISSN)1521-4087 |
| [3] |
Lesnikovich a I I O A, Levchik S V, et al. Thermal decomposition of aminotetrazoles[J]. Thermochim Acta, 2002, 388(1-2): 233-251. DOI:10.1016/S0040-6031(02)00027-8 |
| [4] |
Singh R P, Verma R D, Meshri D T, et al. Energetic nitrogen-rich salts and ionic liquids[J]. Angew Chem, 2006, 45: 3584-3601. DOI:10.1002/(ISSN)1521-3773 |
| [5] |
Klapotke T M. New nitrogen-rich high explosives[J]. Structure and Bonding, 2007, 125: 85-121. DOI:10.1007/978-3-540-72202-1 |
| [6] |
Klapoetke T M, Stierstorfer J, Weber B. New energetic materials: Synthesis and characterization of copper 5-nitriminotetrazolates[J]. Inorganica Chimica Acta, 2009, 362(7): 2311-2320. DOI:10.1016/j.ica.2008.10.014 |
| [7] |
Fischer N, Klapoetke T M, Stierstorfer J. Calcium 5-Nitriminotetrazolate-A green replacement for lead azide in priming charges[J]. Journal of Energetic Materials, 2011, 29(1): 61-74. DOI:10.1080/07370652.2010.505939 |
| [8] |
Semenov S N, Rogachev A Y, Eliseeva S V, et al. 5-Nitroaminotetrazole as a building block for extended network structures: Syntheses and crystal structures of a number of heavy metal derivatives[J]. Polyhedron, 2007, 26(17): 4899-4907. DOI:10.1016/j.poly.2007.06.035 |
| [9] |
Sheldrick G M. SHELXS-97, program for the refining of crystal structure[CP]. Göttingen: University of Göttingen, 1997.
|
| [10] |
Sheldrick G M. SHELXL-97, program for the solution of crystal structure[CP]. Göttingen: University of Göttingen, 1997.
|
Zn(5-NATZ)2(H2O)4 was synthesized by reacting 5-nitraminotetrazolate(5-NATZ) with Zn(NO3)2·6H2O. The single crystal was determined by X-ray single crystal diffraction technology.




