Furoxan derivatives[1-2], which have both high enthalpy of formation, high density and high oxygen balance, lead to an obvious increase in specific impulse of propellant compared to a composition of the same type containing RDX. Especially, 3,4-dinitrofuroxan is a powerful energetic compound first reported in 1993[3]. In addition, it would serve as an intermediate in the synthesis of other furoxan derivatives. Godovikova et al[3-4] reported the synthetic method from glyoxime via two steps of nitration and oxidation (Route A). Furthermore, 3,4-dinitrofuroxan was aslo obtained by treating dinitromethane potassium salt with concentrated H2SO4 or oleum (Route B)[5].But details of two methods were incomplete. In this paper, two approaches of the synthesis of 3,4-dinitrofuroxan were carried out on the basis of literature(scheme 1), the post-processing method for dinitroglyoxime was improved. The structure of ultimate product was well confirmed by 13C NMR, 14N NMR, 15N NMR, IR, MS and elemental analysis, and its 15N NMR and MS spectra were obtained firstly.
|
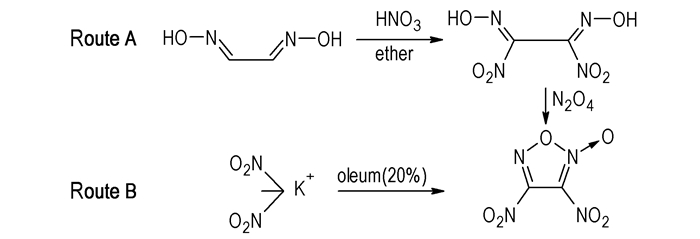
Scheme1 Two synthetic routes of 3,4-dinitrofuroxan |
Route A: To a suspension of 6.6g (0.075 mol) glyoxime and 0.15 g(0.002 mol) NaNO2 in 90mL ether, 56.7 g 25% HNO3(0.225mol) was added dropwise at 15 ℃, then the resulting solution was stirred at 15 ℃ for 2.5 h. The organic layer was separated off, washed with water and dried over anhydrous magnesium sulfate, then filtered and the solvent was removed to give a yellow residue. 20 mL trifluoroacetetic acid was added to the residue. After the solution was cooled to -10 ℃, the yellow precipitate was filtered and dried in air to obtain 7.1 g of solid with yield of 53.2%. 13C NMR(CD3COCD3, 500 MHz), δ: 148.34; 1H NMR(CD3COCD3, 500 MHz), δ: 13.95; IR(KBr, cm-1), υ: 3342, 1665, 1570, 1352 and 835; Anal. Calcd for C2H2N4O6(%): C 13.49, H 1.13, N 31.47; found C 13.78, H 1.13, N 30.19.
A solution of 1.5 mL(25 mmol) N2O4 in 10 mL CCl4 was added dropwise to a suspension of 0.89 g(5 mmol) dinitroglyoxime in 20 mL CCl4 at 0 ℃. After 3.5 h the solvent was removed and the residue was purified by column chromatography on silica gel using a mixture of hexane and ethyl acetate (Rf=0.7, 10:1, V/V) as the eluent, and 0.6 g 3,4-dinitrofuroxan was afforded with yield of 67.4%. 13C NMR(CDCl3, 500 MHz), δ: 119.54, 150.65; 14N NMR(CDCl3, 500 MHz), δ:-40.54(4—NO2), -45.63(3—NO2); IR (KBr, cm-1), υ: 1675, 1581, 1558, 1508, 1461, 1328, 1030, 835; MS(EI) m/z(%): 193(M+OH, 22), 146(M—NO, 24), 130(M—NO2, 100); Anal. Calcd for C2N4O6(%): C 13.60, H 0.00, N 31.80; found C 13.48, H 0.00, N 30.79.
Route B: 2.9 g dinitromethane patassium salt was added to 50 mL oleum(20%) at room temperature, and was stirred for 1 h at 100 ℃, then poured to 100 mL ice-water.The mixture was extracted with ether 50 mL×5. The organic layers were washed with water and dried over anhydrous magnesium sulfate, then filtered and the solvent was removed to give a yellow residue. The title compound was purified by column chromatography on silica gel using a mixture of hexane and ethyl acetate (Rf=0.7, 10:1, V/V) as the eluent, and 0.6 g the title compound was obtained with yield of 17.1%. The analysis show that the compound same as route A.
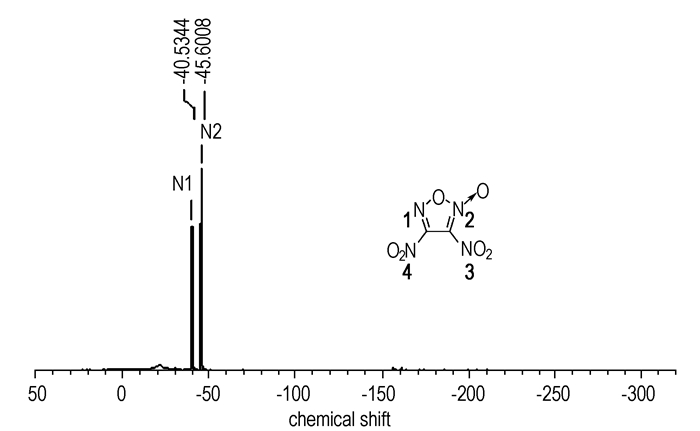
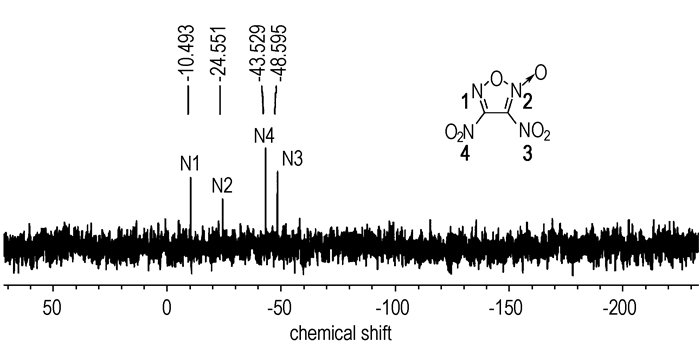
The structure of 3,4-dinitrofuroxan was determined by 13C NMR, 14N NMR, 15N NMR, IR, MS as well as elemental analysis. In the 13C spectra of 3,4-dinitrofuroxan, the resonance bands appear at 119.54 and 150.65. The 14N spectra was showed in Fig. 1, where two signals of N4 and N3 were observed at -40.54 and -45.63 respectively. The 15N spectra of 3,4-dinitrofuroxan (Fig. 2) was depicted with four signals at -10.49(N1), -24.55(N2), -43.53(N4) and -48.60 (N3). The signals of N2, which has a ligand oxygen atom as a neighbor in the furoxan ring, appear as expected at higher field (-24.55) compared with N1(-10.49). In the IR spectra, several main absorption bands around 1675, 1581, 1508, 1461, 1030, 835 cm-1 were attributed to the furoxan ring, and strong absorption bands around 1558, 1328 cm-1 could be assigned to the nitro group.
|
Fig.1 14N spectra of 3,4-dinitrofuroxan |
|
Fig.2 15N spectra of 3,4-dinitrofuroxan |
3,4-Dinitrofuroxan was synthesized from two different routes. Compared with route B the route A allowed the synthesis of 3,4-dinitrofuroxan from accessible initial compounds with a high yield of 35.9%, and its structure was well confirmed by 13C NMR, 14N NMR, 15N NMR, IR, MS and elemental analysis.
| [1] |
Makhova N N, Kulikov A S, Blinnikov A N, et al. 4-Amino-3-azidocar-bonylfuroxan as an universal synton for the synthesis of energetic compounds of the furoxan series[D]//Proc. 29th International ICT-conference, June30-July3, Karsruhe, 1998: 58/1-10.
|
| [2] |
Makhova N N. Synthesis and reactivity of amino-and nitrofuroxans[D]//New Trends in Research of Energetic Materials, Czech Republic, 2011, 25-32.
|
| [3] |
Godovikova T I, Rakitin O A, Golova S P, et al. 3,4-Dinitrofuroxan-the first Example of a Pernitro Heterocycle[J]. Mendeleev Commun, 1993, 3(5): 209-210. DOI:10.1070/MC1993v003n05ABEH000296 |
| [4] |
Godovikova T I, Rakitin O A, Golova S P, et al. Synthesis and nucleophilic substitution reactions of 3,4-Dinitrofuroxan[J]. Chemistry of Heterocyclic Compounds, 1994, 30(4): 465-469. DOI:10.1007/BF01169944 |
| [5] |
Ovchinnlkov I V, Makhova N N, Khmel'nitskll L I. Generation of nitro formonitrile oxide as an intermediate for the preparation of 3,4-dinitrofuroxan[J]. Mendeleev Commun, 1993, 3(5): 210-211. DOI:10.1070/MC1993v003n05ABEH000297 |
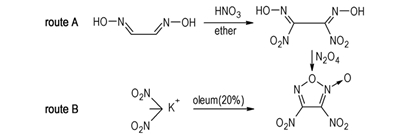
3,4-Dinitrofuroxan was synthesized from two different routes, and its structure was determined by 13C NMR, 14N NMR, 15N NMR, IR, MS as well as elemental analysis.



