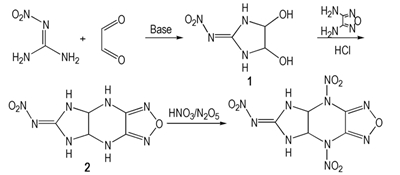
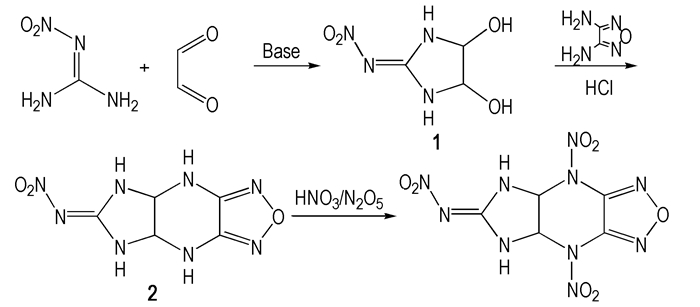
In the last decades, the furazan fused ring compounds have been paid much more attention owing to their characteristics, such as high density, high specific volume and high nitrogen content[1-3]. A novel energetic compound based on furazan fused ring, 6-nitroimino-4,8-dinitro-5,6,7,8-tetrahydro-4H-imidazo [4,5-e] furazano [3,4-b] pyrazine (NIFDNP), was firstly designed and its properties of physico-chemical and detonation were calculated by Gaussian 09 program[4] and VLW method[5]. Using glyoxal and nitroguanidine as starting materials, NIFDNP was synthesized for the first time via the reactions of twice cyclizations and nitrification with a total yield of 14.3% (Scheme 1), NIFDNP and its intermediates were characterized by the means of NMR, IR, MS and EA etc. Furthermore, its melting point was tested by melting point apparatus.
|
Scheme1 The synthetic route of NIFDNP |
Glyoxal (40% in water, 27.9 g, 0.2 mol), 13 mL water were mixed and stirred at room temperature. To this mixture, Na2CO3 (0.48 g, 0.0045 mol) was added, and then nitroguanidine (20.8 g, 0.2 mol) was added in batches after Na2CO3 dissolved completely, the mixture was stirred for another 4 h. The pink precipitate was filtered to obtain 26.2 g solid with a yield of 80.9% and a purity of 99.9% (HPLC). IR (KBr, cm-1) υ: 3375, 3323 (—OH), 3220, 3130 (—NH), 1602 (—C=N), 1560, 1339 (—NO2), 1361 (—CH), 1045 (—C—O); 13C NMR (DMSO-d6, 125 MHz), δ: 160.95 (C=N—NO2), 85.38(CH); Anal. calcd for C3H6O4N4: C 22.23, H 3.73, N 34.56; found C 22.03, H 6.68, N 34.17.
2.2 Synthesis of 6-nitroimino-5,6,7,8-tetrahydro-4H-imidazo[4,5-e] furazano[3,4-b] pyrazine (2)1 (8.1 g, 0.05 mol), 50 mL distilled water were transferred into a three-necked round-bottomed flask with a mechanical stirrer, then 37% hydrochloric acid (35 mL, 0.4 mol) was added dropwise. After warmed to 60 ℃, 1 was dissolved completely, 3,4-diaminofurazano (5.0 g, 0.05 mol) was added in batches and the mixture was stirred for another 2 h at this temperature. The solution was cooled to 10 ℃, then, the orange precipitate was filtered to obtain 9.0 g solid with a yield of 64.5% and a purity of 99.2% (HPLC). IR (KBr, cm-1) υ: 3350, 3221 (—NH), 1639 (—C=N), 1396 (—CH), 1584, 1396 (—NO2) 1621, 1548, 1085 (furazan); 1H NMR(DMSO-d6, 500 MHz) δ: 5.438 (2H, s, CH), 7.868 (2H, s, NH-ipiperazine), 9.128 (2H, s, NH-imidazolidin); Anal. calcd for C5H6O3N8: C 26.55, H 2.67, N 49.55; found C 26.45, H 2.68, N 49.93.
2.3 Synthesis of 6-nitroimino-4,8-dinitro-5,6,7,8-tetrahydro-4H-imidazo[4,5-e] furazano [3,4-b] pyrazine (NIFDNP)A solution of N2O5 (1.8 g, 0.017 mol) in 6 mL 100% nitric acid was placed in a 50 mL three-necked round-bottomed flask with a magnetic stirrer. This was cooled to -5℃ and stirred while 2 (0.4 g, 0.0018 mol) was added in small portion over 5 min. The mixture was allowed to 0-5 ℃ over 5 h. Then the reaction mixture was poured into methylene chloride (60 mL) and cooled to -10 ℃ over 12 h. The white precipitate was filtered to obtain 0.2 g solid with a yield of and 27.4% and a purity of 98.9 % (HPLC). IR (KBr, cm-1) υ: 3432, 3281(—NH), 1617, 1590, 1356 (furazan), 1400 (—CH), 1532, 1334, 1356 (—NO2); 13C NMR(Acetone-d6, 125 MHz) δ: 160.95(C=N—NO2), 141.86(CH), 70.02(C-furazan); Anal. calcd for C5H4O7N10: C 18.99, H 1.27, N 44.30;found C 18.56, H 1.44, N 44.20; MS(EI) m/z: 315 (M-1).
2.4 The physicochemical and detonation performance of NIFDNPThe properties, such as density, enthalpy of formation, detonation velocity and detonation pressure for NIFDNP were shown in the Table 1. As seen in Table 1, NIFDNP has good detonation performances and physicochemical characteristics.
| Tab.1 The properties of NIFDNP |
A new furazan fused ring energetic compound, NIFDNP, was first synthesized with a total yield of 14.3%. In addition, the target compound was found to have good detonation and physicochemical characteristics through calculation and test; which can result in extensive potential applications on gas generator, explosive mixture and propellants.
| [1] |
LI Zhan-xiong. Synthesis, Characterization and Performances of furazan and furoxan energetic materials[D]. Beijing: Beijing University of Science and Technology, 2001.
|
| [2] |
LI Jia-rong. Development of furazan energetic materials[J]. Chinese Journal of Explosives and Propellants, 1998, 21(3): 56-59. |
| [3] |
LI Zhan-xiong, TANG Song-qing, LIU Jin-tao, et al. Synthesis of the derivatives of 1, 4, 5, 8-tetrazanaphthano (2, 3, -6, 7) bisfurazan[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao), 2003, 11(2): 88-90. |
| [4] |
Frisch M J, Trucks G W, Schlegel H B, et al. GAUSSIAN 98 (Revision A. 11)[D]. Gaussian, Inc, Pittsburgh, PA, 2001.
|
| [5] |
WU Xiong, LONG Xin-ping, HE Bi, et al. The VLW equation of state for detonation products[J]. Science in China, 2008, 38(12): 1129-1131. |