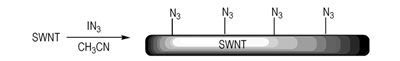
Introducing the energetic groups to the surface of C60 and CNT, attracts special attentions because it might provide a new method for constructing energetic and stable high energy density materials(HEDMs). Peng R F[1] prepared the nitro-modified C60 showing an insensitive action of HMX. However, the research was rare, and many other energetic groups, such as azides were not used despite theirs extensive applications in energetic materials. Therefore, we reported an one-step preparation of the azide-functionalized single-walled carbon nanotubes(SWNTs) (see in scheme 1) according to reference [2].And we characterized it with XPS and FTIR-ATR spectroscopy.

|
Scheme1 Reaction pathway for preparing azide-functionalized nanotubes |
Iodine azide was prepared by a typical reaction of sodium azide and iodine monochloride[3]. 0.2 g sodium azide was added to 20 mL acetonitrile at 0 ℃ and stirred until the solution became ice-cream. Then 0.1 g iodine monochloride was slowly added to the sodium azide solution. Reaction was carried out at 0 ℃ in 20 min before warming to room temperature. The sodium chloride and excessive sodium azide were filtrated to obtain the glassy yellow solution of iodine azide. IR(KBr cm-1): 2253, 2000(—N3), 1375, 1039. Since neat IN3 is explosive, the high concentrations of IN3 solution may enhance the probability of explosion owing to the solid IN3 production.
0.05 g pristine single-walled carbon nanotubes(SWNTs) with an average diameter of 1-2 nm, purchased from XF NANO inc. prepared by CVD method, were dispersed in the solution of iodine azide under stirring and reacted for 0.5 h at room temperature. The dispersion was filtrated with 0.45 um-pore polytetrafluoroethylene (PTFE) membrane filter under vacuum and washed extensively by water and methanol, and dried under vacuum.
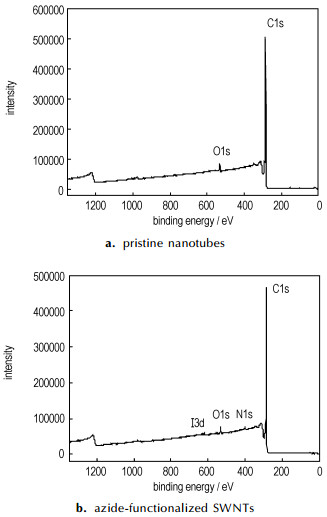
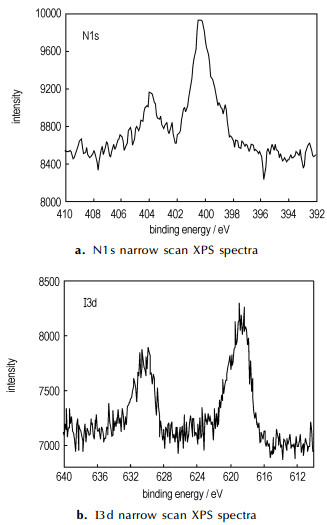
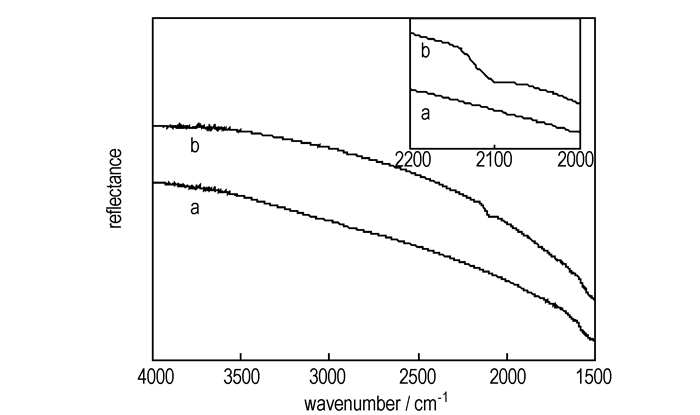
Fig. 1-Fig. 3 are XPS and FTIR-ATR spectroscopy of pristine nanotubes and azide-functionalized carbon nanotubes repectively. Table 1 is the element analysis data according to XPS spectra. Fig. 1 showed that the azide group was attached to nanotubes and the content of N was about 1.6 at.% (see in the Table 1). High-resolution XPS N1s in Figure 2a revealed the two distinct peaks with a peak area ratio of 2-1 which are located at ~400 eV and ~404 eV. The higher energy peak at ~404 eV arises from the central, electron-deficient N in azide group and the peak at ~400 eV could be assigned to the N bonded to nanotubes and the terminal N. The similar XPS peaks were observed on gold surface[4] and silicon(111) surfaces functionalized by azide [5]. The N3 attachment was also characterized by FTIR-ATR spectroscopy. The 2097cm-1 peak corresponds to double bond(N=N=N) of the azide group on the surface(Figure 3).
|
Fig.1 XPS spectra of pristine nanotubes and azide-functionalized SWNTs |
|
Fig.2 N1s and I3d narrow scan XPS spectra of azide-functionalized carbon nanotubes |
|
Fig.3 FTIR-ATR spectra of pristine nanotubes(a) and azide-functionalized carbon nanotubes(b) |
| Tab.1 XPS surface element analysis on the pristine SWNTs and azide-functionalized SWNTs |
It is supposed that the azide groups are introduced into the carbon nanotubes via the addition of IN3 and subsequent elimination of HI. The similar reaction mechanism of azide-modified graphitic is predicted by Hassner[3]. The content of iodine atoms calculated by XPS was only about 6 % of the surface coverage of N atoms(Figure 2b), which was similar to the graphitic surfaces after treatment of IN3[2].
In conclusion, the azide functionalized carbon nanotubes have been prepared and the azide groups characterized by XPS and IR. The nitrogen content has been calculated by XPS to be about 1.6.
| [1] |
Peng R F, Li Y Q, Shu Y J, et al. Synthesis of a fullerene drivative with energy-producing groups[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao),, 2004(suppl): 66-68. |
| [2] |
Devadoss A, Chidsey C E D. Azide-Modified graphitic surface for covalent attachment of alkyne-terminated molecules by "click" chmistry[J]. J Am Chem Soc,, 2007, 129(17): 5370-5371. DOI:10.1021/ja071291f |
| [3] |
Hassner A. Regiospecific and stereospecific introduction of azide functions into organic molecules[J]. Acc Chem Res,, 1971, 4: 9-16. DOI:10.1021/ar50037a002 |
| [4] |
Collman J P, Devaraj N K, et al. Mixed azide-terminated monolayers: a platform for modifying electrode surfaces[J]. Langmuir,, 2006, 22(6): 2457-2464. DOI:10.1021/la052947q |
| [5] |
Cao P, Xu K, Heath J R. Azidation of Silicon(111) Surfaces[J]. J Am Chem Soc, 2008, 130(45): 14910-14911. DOI:10.1021/ja804448p |
Azide groups were attached to single-walled carbon nanotubes. XPS and FTIR-ATR were applied to characterized the surfaces of carbon nanotubes. Results show that the content of N on the SWNTs was about 1.6 at.%.




