The new, insensitive, energetic materials that contain aromatic nitrogen-containing heterocyclic core units have attracted lots of attention due to their relatively high energetic performance, good oxygen balance, density, and other thermodynamic properties when compared to their carbon-only analogous aromatic compounds[1-5].
Imidazole derivatives with more than two nitro groups have been predicted to be high energetic, but insensitive explosives [6-10]. Cho et al. [6] reported that 1-methyl-2, 4, 5-trinitroimidazole was a promising candidate as an insensitive high explosive, with explosive performance comparable to hexogen (RDX). H.S. Jadhav and co-workers[3] reported the synthesis and characterization of N-methyl, N-ester and N-picryl derivatives of 2, 4, 5-trinitroimidazole using triiodoimidazole as substrate. R. Duddu and co-workers[7] developed a new, molten-state nitration method for the synthesis of 1-methyl-2, 4, 5-trinitroimidazole.
4, 5-Dinitroimidazole crystallizes with two crystallographically unique molecules in the monoclinic space group P21/n with unit cell parameters a=11.5360(8)Å, b=9.071(1)Å, c=11.822(1)Å, Z=8, and has a density of 1.781 g·cm-3 [11]. It is well known that insertion of picryl group into an organic compound increases the density of the compound[3]. The titled compound was first obtained by the reaction of picryl fluoride (expensive and difficult to obtain) with 4, 5-dinitroimidazole in DMF in literature [12], and the impact sensitivity (H50) was 50.2 cm. However, few studies were found on the thermodynamic properties of these imidazole derivatives. Therefore, 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole was synthisized by picry chloride, which was much cheaper than picryl fluoride. And the thermal behaviors and single crystal structure of the target compound was studied. Furthermore, the nitrogen equivalent equation (NE equation)[13] was used to predict its detonation velocity and pressure.
2 Experimental 2.1 Materials and analytic instruments4-Nitroimidazole was purchased from YanCheng Medical Chemical Factory. Picryl chloride was obtained according to Ref. [14]. 4, 5-dinitroimidazole was obtained by nitration of 4-nitroimidazole[15]. Melting point was measured on a X-4 melting point apparatus and was uncorrected. The IR spectra was determined on Prestige-21 FTIR spectrophotometer. 1H NMR spectra was recorded on a Bruker Advance 500-MHz-NMR spectrometer. Differential Scanning Calorimetry(DSC) was carried out by Perkin-Elmer DSC-823e. Thermogravimetry (TG) was carried out using TGA/ SDTA851e simultaneous thermal analyzer.
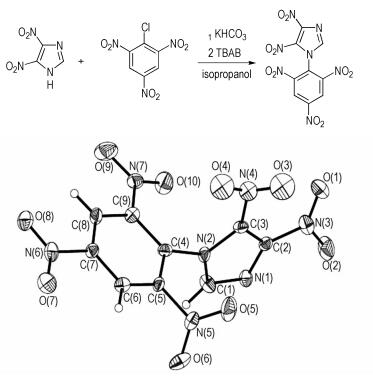
2.2 Synthesis of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazoleA mixture of dinitroimidazole (0.32 g, 2.02 mmol), KHCO3 (0.20 g, 2.00 mmol) and isopropanol (20 mL) was stirred at ambient temperature for 1h, followed by TBAB (0.12 g, 0.38 mmol), then picryl chloride (0.5 g, 2.02 mmol) added in small lots with stirring. The contents were kept stirring for 24 h at 85 ℃. The reaction mixture was filtered and washed with methanol to yield pure product.
Yield: 50%; m.p. 226-229 ℃; IR (υmax, cm-1): 3121 (—CH), 3071 (—CH), 1620, 1538 (C—NO2), 1496, 1467, 1350 (C—NO2), 1338, 1309, 1233, 1186, 1095, 831, 723; 1H NMR (DMSO-d6, 500 MHz) δ: 9.3811 (s, 2H), 8.5897 (s, 1H).
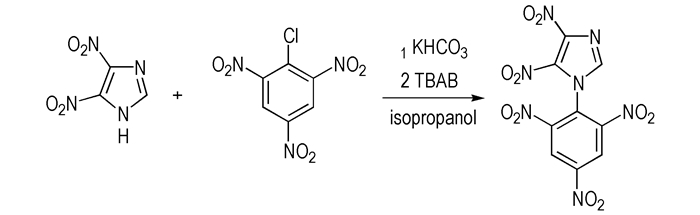
|
Scheme1 Synthesis route of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
The enthalpy change and mass change measurements were made using about 0.6 mg sample in platinum-iridium thermocouples with the temperature ranging from 150 ℃ to 450 ℃ in static air against equal amount of calcined alumina. The heating rate was 10 ℃·min-1. Pt-Ir (Rh 10%) thermocouple assembly was used for ΔT and temperature measurement. For DSC studies, 0.6~0.7 mg sample was crimped in an aluminium cup which was heated against crimped blank cup at different heating rates, i.e. 10, 15, 20, 25 ℃·min-1. Activation energy of the compound was calculated using Ozawa[15] and Kissinger[16] methods.
3 Results and discussion 3.1 Crystal structure description of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazoleA perspective view of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole molecule is shown in Fig. 1, and the three-dimensional packing diagram of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole crystal cell is shown in Fig. 2. Its crystal structure belongs to the orthorhombic system, space group P212121, a=8.2370 (16)Å, b=12.791(3)Å, c=12.916(3)Å, Z=4, V= 1360.8(5)Å3, d=1.802 g·cm-3. Further information concerning the crystal structure determinations in CIF format is available from the Cambridge Crystallographic Data Centre (CCDC-884548). The atomic coordinates, displacement parameters, selected bond distances and angles were listed in Tables 1-3, respectively. All results were obtained by Mercury program.
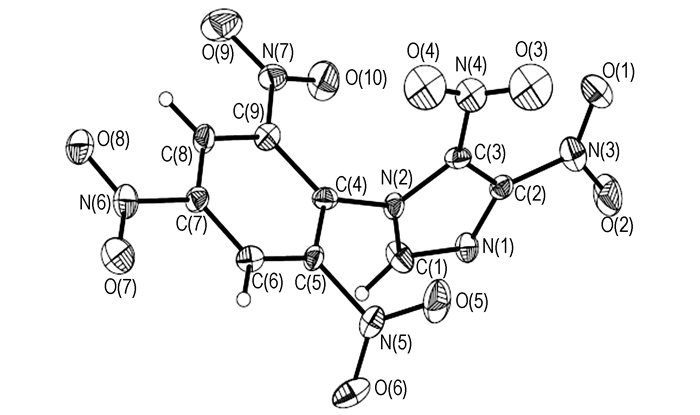
|
Fig.1 Molecular structure of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
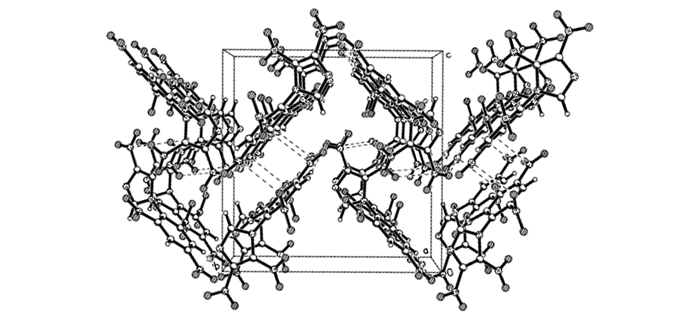
|
Fig.2 Packing diagram of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
| Tab.1 Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2) for 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
| Tab.2 Selected bond lengths (Å) of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
| Tab.3 Selected bond angles (°) of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
There are one endothermic peak and one exothermic peak in the DSC curve of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole (Fig. 4) according to the two obvious mass-loss stages in the TG-DTG curves (Figure 3). The endothermic peak (with peak temperature of 228.98 ℃) in the DSC curve indicates the melting of the compound, which can be confirmed by the first mass-loss stage (in the temperature range of 200-230 ℃) in the TG-DTG curves with mass-loss of 6%. The exothermic peak (with peak temperature of 339.73 ℃) represents the decomposition processes of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole, according to the second mass-loss stage (in the temperature range of 230-330 ℃) in the TG-DTG curves with mass-loss of 87%, the mass loss due to overall reaction (200-440 ℃) is 98%. In order to determine the energy of activation, the DSC studies have been carried out at four different heating rates, i.e. 10, 15, 20, 25 ℃·min-1. The temperatures of decomposition were shown with the heating rate in Table 4. The energy of activation was calculated using Ozawa and Kissinger equations and it is found to be 146.32 kJ·mol-1 and 143.53 kJ·mol-1, respectively, with an excellent linear correlation.
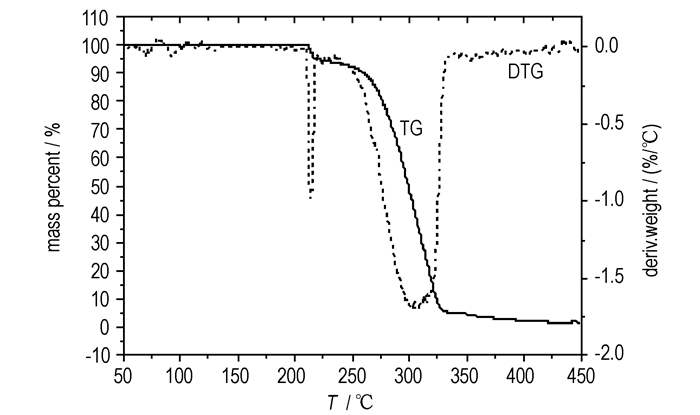
|
Fig.3 TG/DTG curves of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |

|
Fig.4 DSC curves of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole |
| Tab.4 Activation energy according to maximal exothermic peaks at different heating rates |
Detonation velocity (D, m·s-1) and detonation pressure (p, GPa) are the most important targets of the detonation characteristics of energetic materials. The detonation velocity and pressure of an explosive can be predicted with the nitrogen equivalent equation (NE equation) shown as Eq.(1)-(3)[13].
| $ D = (690 + 1160{\rm{ }}\rho {_0})\sum N $ | (1) |
| $ p = 1.092{({\rm{ }}\rho {_0}\sum N)^2} - 0.574 $ | (2) |
| $ \sum N = (100\sum {x_i}{N_i})/M $ | (3) |
where ρ0 represents density of an explosive, g·cm-3, ∑N represents nitrogen equivalent of the detonation products, Ni is nitrogen equivalent index of certain detonation product, xi is the mole number of certain detonation product produced by a mole explosive.
The detonation products produced by general explosives together with their nitrogen equivalent indexes are listed in Table 6. According to the order of H2O—CO—CO2 in forming detonation products, the detonation products of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole are calculated as follows:
| $ {{\rm{C}}_{\rm{9}}}{{\rm{H}}_{\rm{3}}}{{\rm{N}}_{\rm{7}}}{{\rm{O}}_{{\rm{10}}}}{\rm{ = 1}}{\rm{.5}}{{\rm{H}}_{\rm{2}}}{\rm{O + 8}}{\rm{.5CO + 0}}{\rm{.5C + 3}}{\rm{.5}}{{\rm{N}}_{\rm{2}}} $ |
| Tab.6 Nitrogen equivalents of different detonation products |
According to Eq.(3), in which M=369.18, ρ0=1.802 g·cm-3, total nitrogen equivalents of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole are obtained through the nitrogen equivalent indexes of the detonation products in Table 6.
| $ \begin{array}{l} \sum N = {\rm{ }}100 \times (1.5 \times 0.54 + 8.5 \times 0.78 + 0.5 \times 0.15 + \\ 3.5 \times 1)/369.18 = 2.984\\ D = (690 + 1160{\rm{ }}\rho {_0})\sum N = \left( {690 + 1160 \times 1.802} \right) \times 2.984\\ = 8296.48{\rm{ m\cdot}}{{\rm{s}}^{ - 1}}\\ p = 1.092{({\rm{ }}\rho {_0}\sum N)^2} - 0.574 = 1.092 \times {\left( {1.802 \times 2.984} \right)^2} - 0.574\\ = 31.00{\rm{ GPa}} \end{array} $ |
As indicated above, the calculated detonation velocity and pressure of 1-(2′, 4′, 6′-trinitrophenyl)-4, 5-dinitroimidazole are 8296.48 m·s-1 and 31.00 GPa, respectively.
4 Conclusions1-(2′, 4′, 6′-Trinitrophenyl)-4, 5-dinitroimidazole was obtained by the reaction of picryl chloride with 4, 5-dinitroimidazole under KHCO3/TBAB catalytic system, and its melting and decomposition temperatures are 228.98 ℃and 339.73 ℃ and the mass loss of overall reaction (200~440 ℃) is 98%. The calculated crystal density is 1.802 g·cm-3, and the corresponding detonation velocity and pressure are 8296.48 m·s-1 and 31.00 GPa, respectively.
| [1] |
Pagoria P F, Lee G S, Mitchell A R, et al. A review of energetic materials synthesis[J]. Thermochimica Acta, 2002, 384: 187-204. DOI:10.1016/S0040-6031(01)00805-X |
| [2] |
Badgujar D M, Talawar M B, Asthana S N, et al. Advances in science and technology of modern energetic materials: An overview[J]. J Hazard Mat, 2008, 151: 289-305. DOI:10.1016/j.jhazmat.2007.10.039 |
| [3] |
Jadhav H S, Talawar M B, Sivabalan R, et al. Synthesis, characterization and thermolysis studies on new derivatives of 2, 4, 5-trinitroimidazoles: Potential insensitive high energy materials[J]. J Hazard Mat, 2007, 143: 192-197. DOI:10.1016/j.jhazmat.2006.09.014 |
| [4] |
Duddu R, Dave P R, Damavarapu R, et al. Nucleophilic Substitution Reactions of 1-Methyl-2, 4, 5-trinitroimidazole(MTNI)[J]. Synthetic Communications, 2009, 39(23): 4282-4288. DOI:10.1080/00397910902898635 |
| [5] |
Duddu R, Dave P R, Damavarapu R, et al. Synthesis of N-amino-and N-nitramino-nitroimidazoles[J]. Tetrahedron Letters, 2010, 51: 399-401. DOI:10.1016/j.tetlet.2009.11.046 |
| [6] |
Cho J R, Kim K J, Cho S G, et al. Synthesis and characterization of 1-methyl-2, 4, 5-trinitroimidazole (MTNI)[J]. Journal of Heterocyclic Chemistry, 2002, 39(1): 141-147. DOI:10.1002/jhet.v39:1 |
| [7] |
Duddu R, ZHANG Mao-xi, Damavarapu R, et al. Molten-state nitration of substituted imidazoles: New synthetic approaches to the novel melt-cast energetic material, 1-methyl-2, 4, 5-trinitroimidazole[J]. Synthesis, 2011, 17: 2859-2864. |
| [8] |
GAO Hai-xiang, YE Cheng-feng, Gupta O D, et al. 2, 4, 5-Trinitroimidazole-based energetic salts[J]. Chem Eur J, 2007, 13: 3853-3860. DOI:10.1002/(ISSN)1521-3765 |
| [9] |
LI Xiao-hong, ZHANG Rui-zhou, ZHANG Xian-zhou. Computational study of imidazole derivative as high energetic materials[J]. J Hazard Mat, 2010, 183: 622-631. DOI:10.1016/j.jhazmat.2010.07.070 |
| [10] |
SU Xin-fang, CHENG Xin-lu, MENG Chuan-min, et al. Quantum chemical study on nitroimidazole, polynitroimidazole and their methyl derivatives[J]. J Hazard Mat, 2009, 161: 551-558. DOI:10.1016/j.jhazmat.2008.03.135 |
| [11] |
Bracuti A J. Crystal structure of 4, 5-dinitroimidazole[J]. J Chem Crystallogr, 1998, 28(5): 367-371. DOI:10.1023/A:1022412208854 |
| [12] |
Wilson W S. 1-(2', 4', 6'-Trinitrophenyl)imidazoles and -1, 2, 4-triazoles as energetic materials[R]. Naval Air Warfare Center Weapons Div China Lake, Nawcwpns TP8188, AD-A278447, 1994.
|
| [13] |
GUO Yu-xian, ZHANG Hou-sheng. Nitrogen equivalent (NE) and Modified nitrogen equivalent (MNE) equations for prediction Detonation parameters of explosives-prediction of detination velocity of explosives[J]. Explos Shock Waves, 1983, 3(3): 56-66. |
| [14] |
ZHANG Xue-mei, DONG Hai-shan, ZHOU Zhi-ming, et al. A new synthetic route to 1, 3-diamino-5-methylamino-2, 4, 6-trinitrobenzene[J]. Chin J of Energ Mater, 2009, 17(5): 523-526. |
| [15] |
YANG Guo-chen, LIU Hui-jun, CAO Duan-lin. Prepration of 4, 5-nitroimidazol[J]. Chin J of Energ Mater, 2006, 14(5): 349-351. |
| [16] |
Ozawa T. Kinetics in differential termal analysis[J]. Bull Chem Soc Jpn, 1965, 38: 1881-1886. DOI:10.1246/bcsj.38.1881 |
| [17] |
Kissinger H E. Reaction kinetices in differential thermal analysis[J]. Anal Chem, 1957, 29(11): 1702-1706. DOI:10.1021/ac60131a045 |
1-(2′, 4′, 6′-Trinitrophenyl)-4, 5-dinitroimidazole was prepared, and its thermal behavior was studied by DSC and TG.




