Heterocyclic-based compounds, such as pyrazole, triazole, tetrazole, tetrazine, furazan and furoxan derivatives, have most often been utilized in energetic compounds due to their higher positive heat of formation, density, nitrogen content, oxygen balance and better thermal stability than those of their carbocyclic analogues, which are attributed to their large number of C—N, N—N, CᆖN and NᆖN bonds[1-8]. Combustion products of heterocyclic-based compounds contain large amounts of nitrogen, which is environmentally friendly. So, a number of heterocycle-based energetic compounds were reported and extensively used as high-energy explosives and ingredients of propellants[4, 9]. Some of nitrogen-rich energetic compounds were synthesized using the
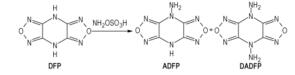
In this paper, two nitrogen-rich energetic compounds, 4-aminodifurazano[3, 4-b, e]pyrazine (ADFP) and 4, 8-diaminodifurazano[3, 4-b, e]pyrazine (DADFP), were synthesized using 4, 8-dihydrodifurazano[3, 4-b, e]pyrazine (DFP) as start material via
Melting points were determined using an open capillary tube. The IR spectra were recorded by NEXUS 870-based Fourier infrared spectrometer employing KBr pellet.
DFP was self-synthesized; hydroxylaminosulfuric acid, hydrochloric acid, sodium carbonate, sodium bicarbonate were AR grade, purchased from Chengdu Kelong Chemical Reagents Factory.
2.2 Synthetic route
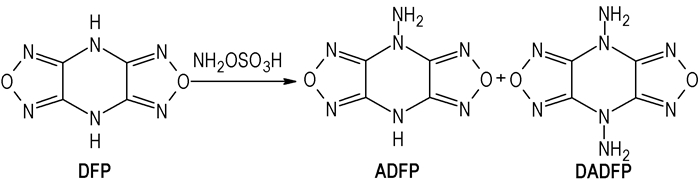
|
Scheme1 Synthetic route of ADFP and DADFP |
To a solution of 0.5 g(0.003 mol) DFP and 0.95 g(0.009 mol) sodium carbonate in 14.5 mL water at 70~75 ℃, a solution of 1.53 g(0.012 mol) hydroxylaminosulfuric acid in 6.5 mL water was added for 10 min. The pH was kept at 8~9 by the addition of sodium bicarbonate. The mixture was kept at 70~75 ℃ for 2 h and then cooled to 15~20 ℃. The precipitate was filtered off, washed with water and acetone, and dried to give 0.11 g off white solid, yield 18.6%. m.p.: 282.3~283.8 ℃.
The above alkaline filtrate was collected, and then the pH value of filtrate was neutralized to 1~2 with hydrochloric acid under the condition of cooling with ice water. The white precipitate was filtered, washed with water, and 0.153 g white solid was obtained with a yield of 28.1%. m.p.: 217.8~219.4 ℃.
ADFP was soluble in dimethyl sulfoxide, acetonitrile, acetone and methanol, and insoluble in water, diethyl ether and petroleum ether. DADFP was soluble in dimethyl sulfoxide, concentrated sulfur acid and acetonitrile, and insoluble in toluene, water and diethyl ether. The structures of ADFP and DADFP were optimized by Gaussian 09 in order to obtain their stable geometric configuration. The explosive parameters were obtained by VLW equation[12] using density and heat of formation as basic data, which were computed by Gaussian 09[13]. The date were shown in table 1. Data show that ADFP and DADFP have better detonation performances than RDX and high positive heat of formation.
| Tab.1 Properties of ADFP, DADFP and RDX |
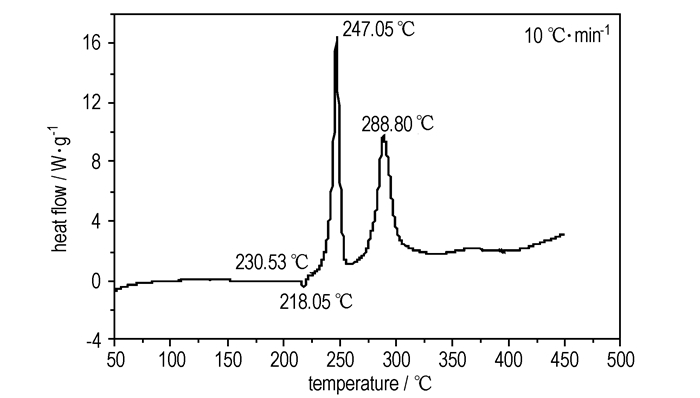
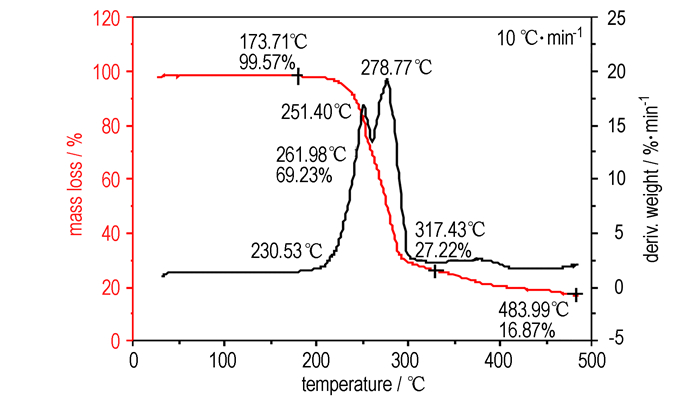
The DSC and TG-DTG analyses revealed that ADFP was thermally stable up to 230.5 ℃. The DSC curve (Fig. 1) exhibited a melting point at 218.1 ℃ and two thermal decomposition peaks at 247.1 ℃and 288.8 ℃, respectively. The TG-DTG curves (Fig. 2) showed that there were two stages in the decomposition process of ADEP with a mass loss of 30.77% before 262.0 ℃ at first stage and a total mass loss of 72.78% before 317.4 ℃, and a 16.87% residue at 484.0 ℃.
|
Fig.1 DSC curve of ADFP |
|
Fig.2 TG-DTG curves of ADFP |
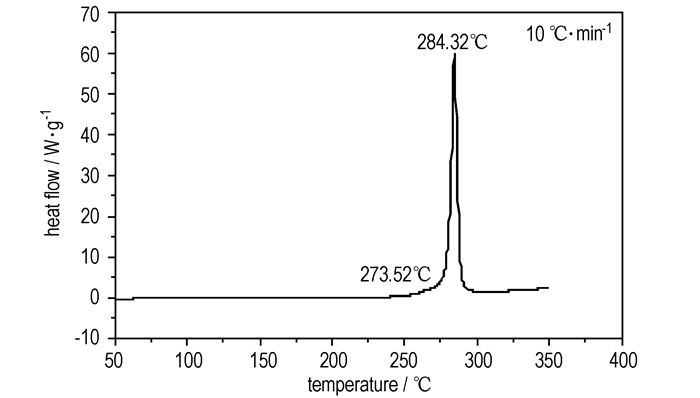
The DSC and TG-DTG analyses revealed that DADFP was thermally stable up to 273.5 ℃. The DSC curve (Fig. 3) exhibited a melting point at 284.3 ℃, and one thermal decomposition peak at 305.7 ℃(Fig. 4). TG-DTG curves (Fig. 4) showed that there was one main decomposition stage with a mass loss of 68.98% before 394.7 ℃, and 24.78% residue at 497.4 ℃.
|
Fig.3 DSC curve of DADFP |
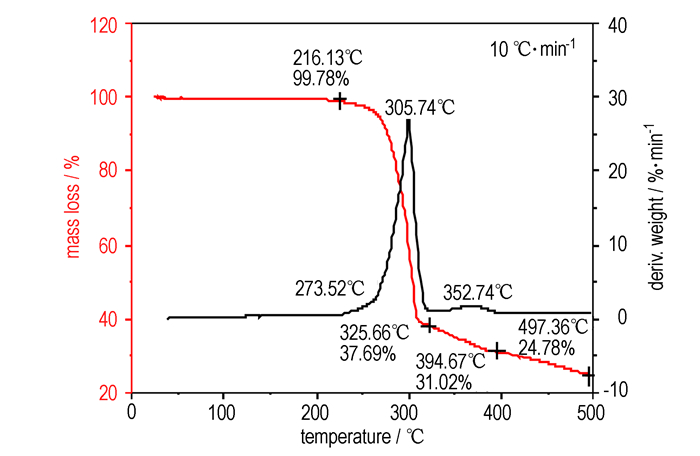
|
Fig.4 TG-DTG curves of DADFP |
(1) Two
(2) ADFP and DADFP have better detonation performances than RDX. Especially, both of them have high positive heat of formation: 1164.47 kJ·
(3) The date indicate that ADFP and DADFP havegood thermal stability.
| [1] |
Singh R P, Gao H X, Meshri D T, et al. Nitrogen-rich heterocycles[J]. Struct Bond, 2007, 125: 35-83. DOI:10.1007/978-3-540-72202-1 |
| [2] |
Klapötke T M. New nitrogen-rich high explosives[J]. Struct Bond, 2007, 125: 85-121. DOI:10.1007/978-3-540-72202-1 |
| [3] |
Klapötke T M, Piercey D G. 1, 1′-Azobis(tetrazole): a highly energetic nitrogen-rich compound with a N10 chain[J]. Inorg Chem, 2011, 50(7): 2732-2734. DOI:10.1021/ic200071q |
| [4] |
WANG Ying-lei, ZHAO Feng-qi, JI Yue-ping, et al. Synthesis and thermal behaviors of 4-amino-3, 5-dinitro-1H-pyrazole[J]. Journal of Analytical and Applied Pyrolysis, 2012, 98: 231-235. DOI:10.1016/j.jaap.2012.08.014 |
| [5] |
ZHOU Qun, WANG Bo-zhou, JIA Si-yuan. New synthetic process of 1-methyl-3-amino-5-nitro-1, 2, 4-triazole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(1): 26-29. |
| [6] |
HE Yun, FAN Gui-juan, ZHANG Guang-Quan, et al. Review on synthesis and reactivity of 5-amino-3-nitro-1, 2, 4-trizole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(6): 715-720. |
| [7] |
ZHANG Ye-gao, WANG Bo-zhou, LIU Qian, et al. Synthesis of 5-(4-nitro-1, 2, 5-oxadiazol-3-yl)-5H-[1, 2, 3] triazolo[4, 5-c][1, 2, 5]oxadiazolium Inner salt[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2010, 18(4): 383-386. |
| [8] |
Li Yu-chun, Qi Cai, Li Sheng-hua, et al. 1, 1′-Azobis-1, 2, 3-triazole: a high-nitrogen compound with stable N8 structure and photochromism[J]. J Am Chem Soc, 2010, 132: 12172-12173. DOI:10.1021/ja103525v |
| [9] |
Thottempudi V, Gao H X, Shreeve J M. Trinitromethyl-substituted 5-nitro-or 3-azo-1, 2, 4-triazoles: synthesis, characterization, and energetic properties[J]. J Am Chem Soc, 2011, 133: 6464-6471. DOI:10.1021/ja2013455 |
| [10] |
Vinogradov V M, Dalinger I L, Shevelev S A. N-amination of pyrazoles: a general approach[J]. Mendeleev Commun, 1993, 3(3): 111. DOI:10.1070/MC1993v003n03ABEH000247 |
| [11] |
Starchenkov I B, Andrianov V G, Mishnev A F. Chemistry of furazano[3, 4-b]pyrazine. 1. synthesis and thermodynamic appraisal of 4, 8-dihydrodifurazano[3, 4-b, e]pyrazine and its derivatives[J]. Chem Heterocycl Compd, 1997, 33(2): 216-228. DOI:10.1007/BF02256764 |
| [12] |
WU Xiong, LONG Xin-ping, HE Bi, et al. The VLW equation of state for detonation products[J]. Science in China, 2008, 38(12): 1129-1131. |
| [13] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09[CP]. Gaussian, Inc, Wallingford CT, 2009.
|
| [14] |
HE Zhi-wei, LIU Zu-liang, WANG Ai-ling. Influence of 2, 6-diamino-3, 5-dinitropyridine-1-oxide on properties of RDX[J]. Chinese Journal of Explosives & Propellants, 2010, 33(1): 11-14. |
Two N-amino energetic derivatives of 4,8-dihydrodifurazano[3,4-b,e]pyrazine (DFP), 4-aminodifurazano[3,4-b,e]pyrazine (ADFP) and 4,8-diaminodifurazano[3,4-b,e]pyrazine (DADFP) were synthesized via N-amination reaction. The properties of ADFP and DADFP were estimated. The main themal properties of ADFP and DADFP were analyzed by DSC and TG techniques.




