The development of new energetic materials continues to focus on the synthesis of new heterocycles with high density, high heat of formation, and good oxygen balance. These materials were shown to be useful as high explosives, components of propellants and gas generators[1-4]. In 1995, 2, 6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105) was synthesized for the first time[5]. It was found that the energy predicted was 125% of the extremely insensitive explosive 2, 4, 6-triamino-1, 3, 5-trinitrobenzene (TATB), 81% of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine (HMX)[6-7], and it was a thermally stable (Tp=342 ℃(DSC)), relatively insensitive energetic material (H50=117 cm). All these characteristics made it very promising for insensitive boosters and detonators.
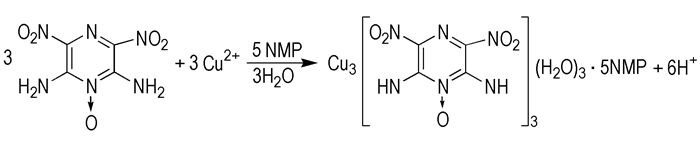
Due to the positive heat of formation and thermal stability, nitrogen heterocyclic rings as ligands to construct energetic coordination compounds have received a great deal of interest[8-13]. Coordination compounds offer significant energetic performance improvement because of the presence of both oxidizing and reductive groups in the same complex molecules. In this paper, an energetic complex [Cu3(C4H2N6O5)3(H2O)3]·5NMP (NMP=N-Methyl pyrrolidone) was obtained and its crystal structure was investigated. Furthermore, thermal decomposition process and the kinetic parameters were investigated. In addition, the catalytic performance toward the thermal decomposition of ammonium perchlorate (AP) was explored.
2 Experiment 2.1 Materials and instrumentsAll chemicals from commercial sources were of analytical pure and used without further purification. LLM-105 was prepared according to the reported method[14].
Elemental analysis were carried out using Vario EL-III analyzer; Infrared (IR) spectra was recorded on Nicolet-10 FTIR; The single crystal X-ray diffraction was performed on Nonius-CAD-4 diffractometer with graphite monochromated Mo Ka radiation (λ=0.071073 nm). Differential Scanning Calorimetry (DSC) study was performed on DSC823e METTLER TOLEDO with heating rates of 5, 10, 15, 20 K·min-1; thermogravimetry (TG) analysis was conducted on TGA/SDTA851e METTLER TOLEDO with a heating rate of 20 K·min-1, in a flow of dry oxygen-free nitrogen at 30 mL·min-1.
2.2 Synthesis of [Cu3(C4H2N6O5)3(H2O)3]·5NMPA mixture containing Cu(CH3COO)2·H2O (0.6 g, 3.0 mmol), LLM-105 (0.648 g, 3.0 mmol) and N-Methyl pyrrolidone (30 mL) was sealed in a 50 mL reactor at 80 ℃ for 2 hours, then cooled to room temperature, filtered, and dried in air, then, the resulting green solid powder (0.781g) was obtained in yield of 56.48%. After keeping the clear filtrate for several weeks, green-block like single crystals were obtained. Anal. Calcd. : C, 32.11; H, 4.12; N, 23.29. Found: C, 32.37; H, 4.35; N, 23.55. IR(KBr) 3321, 3276, 1562, 1535, 1402, 1327, 1225, 1137, 884, 811, 779, 712 cm-1.
|
The structure was solved by direct methods using the SHELXS 97 program[15] and refined by full-matrix least squares on F2 using SHELXL 97 program[16]. All the non-hydrogen atoms were obtained from the difference Fourier map and refined. The hydrogen atoms were obtained from the difference Fourier map or positioned geometrically and treated as riding on the parent atoms or constrained in the locations during refinements. Crystallographic and refinement data were listed in Table. 1.
| Tab.1 Crystallographic data and structure refinement details for [Cu3(C4H2N6O5)3(H2O)3]·5NMP |
Theoretical calculation results show that all the atoms of LLM-105 are in the same plane within which the delocalization of Л electrons exists[17]. The oxygen atom in N→O group is easy to coordinate with transition metals because of small steric hindrance and free lone pair electrons. The nitrogen atom in amino group participates in the hyperconjugation system due to a strong interaction with the lone pair orbital, which decreases the density of electronic cloud. So it is hard for the N atom in amino group to provide additional lone pair electrons to coordinate with metal cations. After heterogenous splitting of N—H bond, each amino group loses a proton to provide one pair of lone electrons to coordinate with central metal atom in the coordination structure[18].
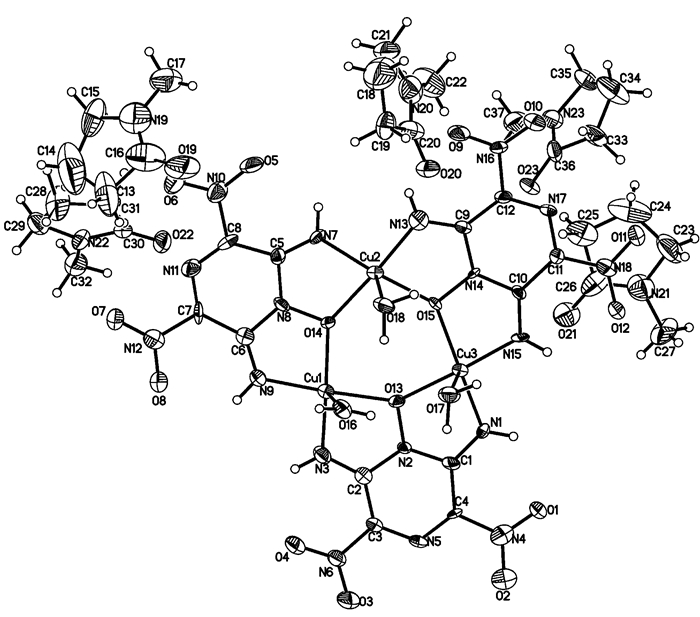
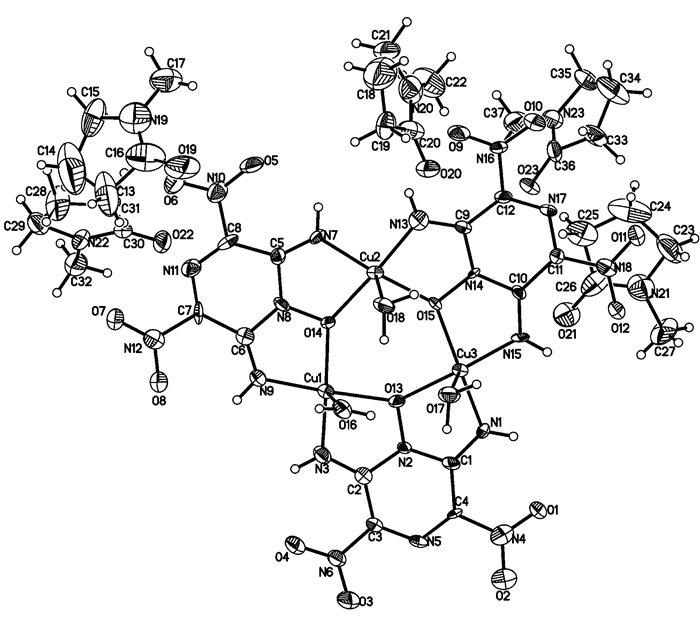
3.2 Structural descriptionA perspective view of the complex is shown in Fig. 1; the selected bond lengths and bond angles of the complex are listed in Table 2.
|
Fig.1 Molecular structure of [Cu3(C4H2N6O5)3(H2O)3]·5NMP |
| Tab.2 Selected bond lengths (nm) and bond angles(°) for[Cu3(C4H2N6O5)3(H2O)3]·5NMP |
As can be seen from Fig. 1, the molecular unit of [Cu3(C4H2N6O5)3(H2O)3]·5NMP is comprised of three central copper cations, three deprotonated LLM-105 anions, three coordinated H2O and five lattice NMP. The coordination number of each copper cation is five and the coordination structure forms distorted tetragonal pyramid. The copper cation directly coordinates with two imine nitrogen atoms and two N→O oxygen atoms from two different LLM-105 ligands, and forms two five-membered chelate rings. The three Cu cations and coordinated O atoms form a distorted six-member ring. The bond distance of Cu—O coordination bonds is from 1.961(4)Å to 2.430(5)Å, Cu—N from 1.897(6)Å to 1.931(6)Å, respectively. It is worth noting that some Cu—O bond distances are considerably longer than others: Cu(1)—O(16), 2.430(5)Å, Cu(2)—O(18), 2.327(5)Å, Cu(3)—O(17), 2.438(5)Å. This means that these coordination bonds are significantly week. As a tridentate ligand, LLM-105 forms two five-membered chelate rings. Each five-membered chelate ring is formed by one copper atom with oxygen atom from N→O group in the LLM-105 ring and nitrogen atom form imine group on the same side. The stability of the five-membered chelate rings and the excellent molecule flexibility make an important contribution to the stability of the complex.
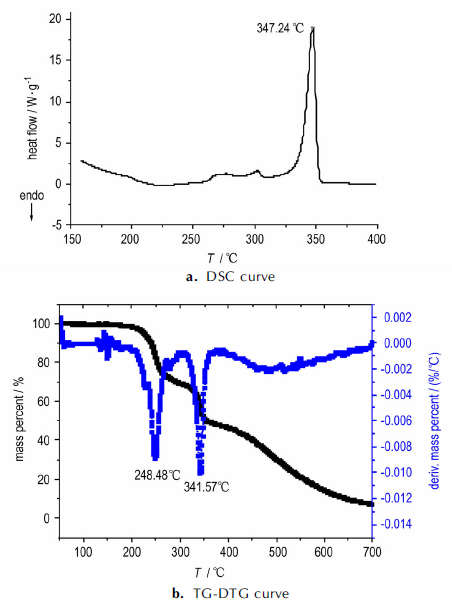
3.3 Thermal decompositionThe DSC and TG-DTG analyses were carried out in order to investigate the thermal behaviors of [Cu3(C4H2N6O5)3(H2O)3]·5NMP. DSC and TG-DTG curves are illustrated in Fig. 2 at 20 K·min-1.
|
Fig.2 DSC and TG-DTG curves of [Cu3(C4H2N6O5)3(H2O)3]·5NMP |
DSC curve shows only one main exothermic peak in 310~360 ℃ at peak temperature of 347.238 ℃. It can be seen from the TG-DTG curves that there are three main successive mass loss stages from 50 ℃ to 700 ℃. The first mass loss stage starts at 70 ℃, ends at about 280 ℃ with mass loss of 35.25%, which is coincident with the theoretical value (35.87%) of losing five lattice NMP molecules. The second stage from 280 ℃ to 380 ℃ which reaches the largest rate at 341.6 ℃ is considered as the ring breaking of LLM-105 with mass loss of 22.17 %. With temperature increasing, the complex continues to lose mass until 700 ℃. The mass fraction of the final residues is 17.24%, coincident with the calculated value(17.36 %)of the end product CuO. So, it can be speculated that the final decomposition product is CuO.
3.4 Non-isothermal reaction kinetics analysisTo explore the thermal decomposition mechanism of the exothermic reaction of [Cu3(C4H2N6O5)3(H2O)3]·5NMP, the kinetics parameters of the exothermic process by the Kissinger′s[19] and Ozawa′s[20-21] methods were explored. The Kissinger equation and Ozawa-Doyle equations are as follows:
| $ \begin{array}{l} \ln \frac{\beta }{{T_{\rm{p}}^2}} = \ln \left( {\frac{{RA}}{E}} \right) - \frac{E}{{RT}}\\ \ln \beta + \frac{{0.4567E}}{{R{T_{\rm{p}}}}} = C \end{array} $ |
Where Tp is the peak temperature, ℃; R is the gas constant, 8.314 J·mol-1·K-1; β is the linear heating rate, ℃·min-1; C is a constant.
Based on the multiple non-isothermal DSC curves obtained at four different heating rates of 5, 10, 15, 20 K·min-1, the values of the apparent activation energy (Ek and E0), the pre-exponential factor (Ak) and linear correlation coefficient (rk and r0) of the two intense exothermic decomposition processes were determined. The detailed data and the calculated kinetic parameters are listed in Table 3.
| Tab.3 Nonisothermal reaction kinetic parameters for [Cu3(C4H2N6O5)3(H2O)3]·5NMP |
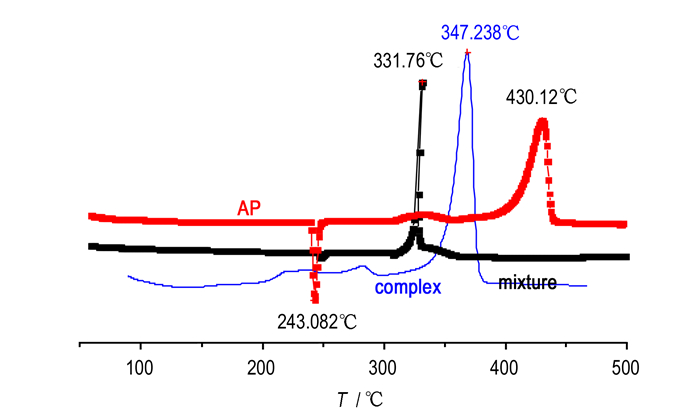
As the main high energy ingredient of solid propellants, AP occupies a large proportion in the formula. The application of AP can improve the performance of propellants to some extent. The thermal decomposition can directly affect the burning velocity and energy features of propellants[22-24]. Therefore, extensive studies on the thermal decomposition of AP were carried out. [Cu3(C4H2N6O5)3(H2O)3]·5NMP with AP was mixed at a mass ratio of 1:3 to prepare the target samples for the thermal decomposition analysis. The DSC curves of pure compounds and the mixture are shown in Fig. 3.
|
Fig.3 DSC curves for pure compounds and mixture at a heating rate of 20 K·min-1 |
From Fig. 3, the thermal decomposition of pure AP can be divided into three stages. The first stage corresponds to the phase transition endothermic process of AP, which transforms from orthorhombic to cubic crystal with peak temperature at 243.08 ℃ ranging from 240 ℃ to 250 ℃. The second stage corresponds to the low-temperature decomposition exothermic process of AP with peak temperature at about 330 ℃ with the heat of 45.93 J·g-1. The third stage corresponds to the higher temperature decomposition exothermic process of AP with peak temperature at 430.12 ℃ with the heat of 486.33 J·g-1 ranging from 380 ℃ to 440 ℃. DSC curve of [Cu3(C4H2N6O5)3(H2O)3]·5NMP shows only one main exothermic peak at peak temperature of 347.238 ℃.
The DSC curve of mixture system shows that the complex with AP have no significant effects on the crystallographic transition temperature around 245 ℃, but some significant changes in the decomposition pattern occur. In the mixture system, it is obvious that the low-temperature exothermic decomposition peak overlaps with the high temperature exothermic decomposition peak 331.757 ℃ of the complex, and it is 98.363 ℃ lower than pure AP at 430.12 ℃. The sharp exothermic peak indicates a rapid decomposition process of the mixture; the decomposition heat of the mixture systems increases dramatically from 486.33 J·g-1 to 1372.38 J·g-1. It is proved that oxygen species is preferably absorbed by the metallic species at heating condition, resulting in the following rapid reaction to form metal oxide with large amount of heat. It is reasonable as considering the huge amount of energy giving rise to the high catalytic activity. Therefore, the complex shows good catalytic performance to the thermal decomposition of AP.
4 ConclusionsIn summary, [Cu3(C4H2N6O5)3(H2O)3]·5NMP was synthesized with a yield of 56.48% and characterized by single-crystal X-ray diffraction. Thermal analysis shows that there is one exothermic decomposition stage from 50 ℃ to 500 ℃ with final residues 17.24%. The catalytic performance of [Cu3(C4H2N6O5)3(H2O)3]·5NMP toward the thermal decomposition of AP was also explored. The complex decreased the higher thermal decomposition temperature of AP by 98.363 ℃, increased the exothermic quantity of decomposition by 886.05 J·g-1, showing apparent catalytic effects on the thermal decomposition of AP.
| [1] |
Meyer R, Kohler J, Homburg A. Explosives[M]. 5th Ed, Wiley-VCH, Weinheim, Germany, 2002.
|
| [2] |
Agrawal J P, Hodgson R D. Organic chemistry of Explosives[M]. Chichester, UK: John Wiley and Sons, 2007.
|
| [3] |
Chen J, Qiao Z Q, Wang L L, et al. Fabrication of rectangular 2, 6-diamino-3, 5-dinitropyrazine-1-oxide microtubes[J]. Materials Letters, 2011, 65: 1018-1022. DOI:10.1016/j.matlet.2011.01.005 |
| [4] |
Sikder A K, Sikder N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications[J]. Journal of Hazardous Materials, 2004, 112: 1-15. DOI:10.1016/j.jhazmat.2004.04.003 |
| [5] |
Pagoria P F. Synthesis of LLM-105[J]. Propellants, Explosive, Pyrotechnics, 1995, 20(1): 38-42. |
| [6] |
Pagoria P F, Cutting J F, Hoffman M D, et al. Energetic materials for munitions[R]. DEA-A-76-G-1218, 1998.
|
| [7] |
Cutting J L, Chau H H, Hodgin R L, et al. A small-scale screening test for H E performance: application to the new explosive LLM-105[R]. UCRL-JC-131623, 1998.
|
| [8] |
Klapotke T M, Stierstorfer J, Weber B. New energetic materials: Synthesis and characterization of copper 5-nitriminotetrazolates[J]. Inorganica Chimica Acta, 2009, 362: 2311-2320. DOI:10.1016/j.ica.2008.10.014 |
| [9] |
Singh G, Felix S P, Studies on energetic compounds 25. An overview of preparation, thermolysis and applications of salta of 5-nitro-2, 4-dihydro-3H-1, 2, 4-triazol-3-one(NTO)[J]. Journal of Hazardous Materials, 2002, 90: 1-17. DOI:10.1016/S0304-3894(01)00349-1 |
| [10] |
SHENG Di-lun, MA Feng-e, SUN Fei-long, et al. Study on synthesis and main properties of BNCP[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2000, 8(3): 100-103. 盛涤伦, 马凤娥, 孙飞龙, 等. BNCP起爆药的合成及其主要性能[J]. 含能材料, 2000, 8(3): 100-103. |
| [11] |
LIU Jin-jian, LIU Zu-liang, CHENG Jian. Crystal Structure and thermal Decomposition Properties of Energetic Complex Ni(C5N5O5H4)2(by)2[J]. Chinese Journal of Explosives and Propellants, 2012, 35(2): 36-39. 刘进剑, 刘祖亮, 成健. 含能配合物Ni(C5N5O5H4)2(by)2的晶体结构和热分解性能[J]. 火炸药学报, 2012, 35(2): 36-39. |
| [12] |
Friedrich M, Galvez-Ruiz J C, Klapotke T M, et al. BTA copper complexes[J]. Inorganic Chemistry, 2005, 44(22): 8044-8052. DOI:10.1021/ic050657r |
| [13] |
Yang Q, Chen S P, Xie G, et al. Synthesis and characterization of an energetic compound Cu(Mtta)2(NO3)2 and effect on thermal decomposition of ammonium perchlorate[J]. Journal of Hazardous Materials, 2011, 197: 199-203. DOI:10.1016/j.jhazmat.2011.09.074 |
| [14] |
Pagoriam P F. Synthesis, scale-up and characterization of 2, 6-diamino-3, 5-dinitropyrazine-1-oxide[J]. Propellants, Explosives, Pyrotechnics, 1998, 23(3): 156-160. |
| [15] |
Sheldrick G M. SHELXS-97, Program for X-ray crystal Structure Determination[CP]. University of Gottingen, Germany, 1997.
|
| [16] |
Sheldrick G M. SHELXL-97, Program for X-ray Crystal Structure Refinement[CP]. University of Gottingen, Germany, 1997.
|
| [17] |
Chen J, Qiao Z Q, Wang L L, et al. Fabrication of rectangular 2, 6-diamino-3, 5-dinitropyrazine-1-oxide microtubes[J]. Matters Letters, 2011, 65: 1018-1021. DOI:10.1016/j.matlet.2011.01.005 |
| [18] |
Yamanouchi K, Enemark J H. Structure of Tris[2-aminobenzenethiolato (2-)-N, S]molybdenum (Ⅵ), Mo(NHC6H4S)3[J]. Inorganic Chemistry, 1978, 17: 2911-2917. DOI:10.1021/ic50188a045 |
| [19] |
Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29: 1702-1706. DOI:10.1021/ac60131a045 |
| [20] |
Ozawa T. A new method of analyzing thermo-gravimetric data[J]. Bulletin of the Chemical Society of Japan, 1965, 38: 1881-1885. DOI:10.1246/bcsj.38.1881 |
| [21] |
Doyle C D J. Kinetic analysis of thermo-gravimetric data[J]. Journal of Applied Polymer Science, 1961, 5: 285-292. DOI:10.1002/app.1961.070051506 |
| [22] |
Sun X F, Qiu X Q, Li L P, et al. ZnO Twin-Cones: Synthesis, photoluminescence, and catalytic decomposition of ammonium perchlorate[J]. Inorganic Chemistry, 2008, 47: 4146-4152. DOI:10.1021/ic702348c |
| [23] |
Duan H Z, Lin X Y, Liu G P, et al. Synthesis of Co nanoparticles and their catalytic effect on the decomposition of ammonium perchlorate[J]. Chinese Journal of Chemical Engineering, 2008, 16: 325-328. DOI:10.1016/S1004-9541(08)60082-8 |
| [24] |
Liu H B, Jiao Q Z, Zhao Y, et al. Mixed oxides derived from Cu-Co layered double hydroxide nanorods: Preparation, characterization and their catalytic activities[J]. Journal of Alloys and Compounds, 2010, 496: 317-323. DOI:10.1016/j.jallcom.2010.02.004 |
An energetic complex with ligand 2, 6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105) was synthesized and characterized. Furthermore, its thermal decomposition processes and the catalytic performance toward thermal decomposition of AP were explored.




