2. School of Pharmacy, China Pharmaceutical University, Nanjing 210009, China
2. 中国药科大学药学院, 江苏 南京 210009
In recent years, the synthesis of energetic, heterocyclic compounds have received a great amount of interest owing to their higher heat of formation, density, and oxygen balance than those of carbocyclic analogues [1-4], especially the nitro derivatives of pyrazines and their analogues for insensitive explosives [5-11]. Pagoria et al. [12-13] synthesized 2, 6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105) by reaction 2, 6-dichloropyrazine with sodium methoxide to yield 2-methoxy-6-chloropyrazine, following by nitration with mixed acid at 70 ℃ to yield 2, 6-diamino-3, 5-dinitropyrazine (ANPZ), and then oxidation to give the target compound, which exhibits a density of 1.918 g·cm-3[14] and decomposition temperature of 354 ℃. This work also illustrated that the conversion of tertiary amines to their corresponding N-oxides can increase density and oxygen balance in heterocyclic systems [15].
As another class of energetic materials, cyclourea nitramines increasingly gain importance as perspective and highly energetic materials. In general, both the mono-and dinitrourea explosives have very high densities (>1.90 g·cm-3) which has been attributed to the inherently high density of the urea framework. However, the dinitrourea explosives suffer from hydrolytic liability, and thus restricting their use. But the mono-nitrourea compounds are fairly stable to hydrolysis and are relatively insensitive to shock [16-19].
With the concept of designing and sythesizing new nitro derivatives of symmetric pyrazino-dicycloureas, 2, 3, 5, 6-tetraaminepyrazine reacts with urea to yield a precursor with two five-membered rings, which provides a possible conversion of tertiary amines to their corresponding N-oxides and more N—H sites for introducing nitro substituents, and thus generates a series of new energetic materials (Scheme 1). Upnow, the study on the relationship between structure and property of nitro derivatives of symmetric pyrazino-dicycloureas is little. Therefore, we report a systematic study on the density, heats of formation, thermal stability, and energetic properties of nitro derivatives of symmetric pyrazino-dicycloureas by density functional theory (DFT) method. Detonation velocities and pressures were predicted using the calculated HOF and densities.
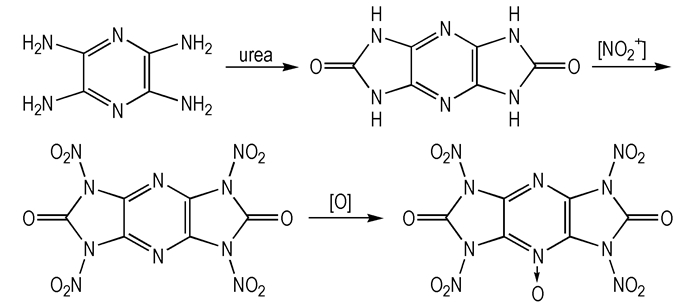
|
Scheme1 |
Calculations were carried out by using the Gaussian 09 program suite. The geometry optimization of the structures and frequency analysis were carried out by using the B3LYP functional with the 6-31G** basis set [20-21]. All of the optimized structures were characterized to be true local energy minima on the potential-energy surface without imaginary frequencies. Isodesmic reactions were designed for the prediction of gas phase heats of formation. Scheme 2 shows a representative isodesmic reaction for compound 6 (see in Scheme 3).
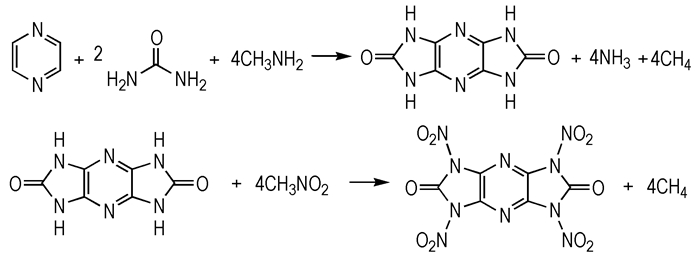
|
Scheme2 |
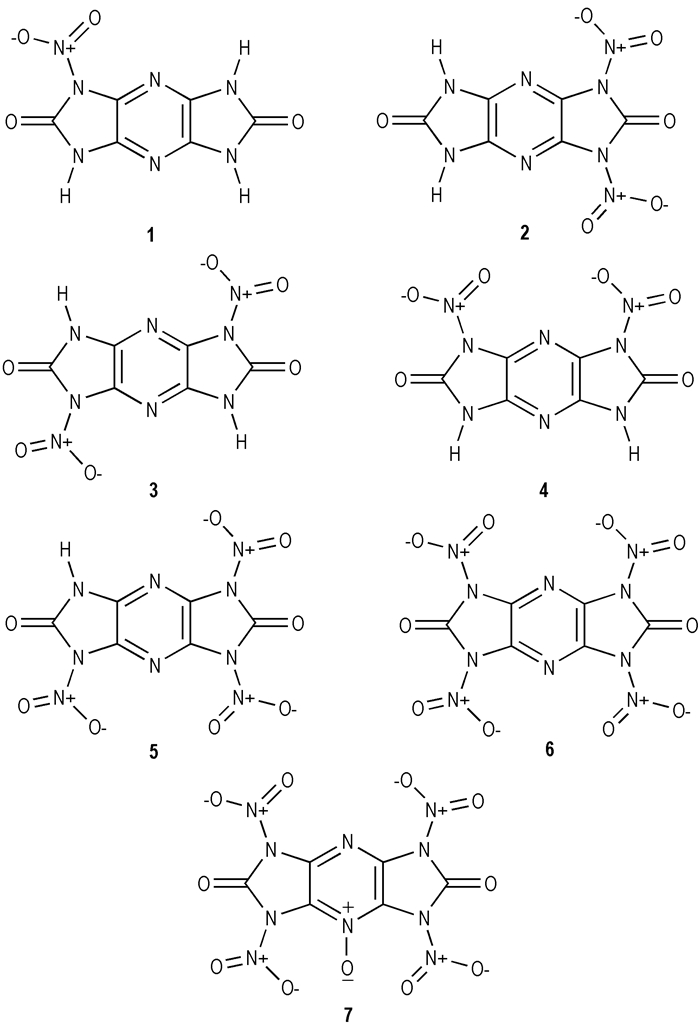
|
Scheme3 Illustration of the molecular structures of the seven title compounds |
For C—H—N—O explosives, the Kamlet and Jacob empirical equations (1) and (2) were used to determine detonation parameters[22].
| $ p = 1.558N{M^{1/2}}{Q^{1/2}}{\rho ^2} $ | (1) |
| $ D = 1.01{(N{M^{1/2}}{Q^{1/2}})^{1/2}}(1 + 1.30\rho ) $ | (2) |
where p is detonation pressure in GPa, D is detonation velocity in km·s-1, N is the number of moles of gaseous detonation products per gram of explosive, M is the average molecular weight of the gaseous products, Q is the energy of the detonation reaction of explosive in J·g-1 and ρ is the crystal density in g·cm-3. N, M and Q are decided according to the largest exothermic principle, i.e., for C—H—N—O explosives, all the N atom convert into N2, the O atom forms H2O with H atom first and the remainder forms CO2 with C atom. The remainder of C atom will exist in solid state if O atom does not satisfy full oxidation of C atom. The remainder of O atom will exist in O2 if O atom is superfluous. Table 1 presents the methods for calculating the parameters N, M, and Q of CaHbOcNd explosives[23].
| Tab.1 Methods for calculating the N, M, and Q parameters of CaHbOcNd explosives |
At the outset, we have performed structure optimizations of compounds 1-7 in Scheme 3 at the B3LYP/6-31G** level, and selected optimized bond lengths of nitro derivatives of symmetric pyrazino-dicycloureas are in Table 2, and corresponding dihedral angles are listed in Table 3. Investigations on the optimized geometries, variations (i.e. the differences between the maximum and minimum values) of the calculated results for the C—C, C—N, and N—N bond lengths and N—C—N, C—N—C, and C—N—N angles are much more different from all title compounds, indicating that these geometrical parameters are more sensitive to the environmental or molecular structures. The N(11)—N(15) bond length of compound 1 is 1.427 Å which can be used as a original bond length with an introduction of one nitro group. The N—NO2 bond lengths of compounds 2, 3, and 4 are 1.443, 1.432, 1.436 Å, respectively. From the results, both N—C bonds (e.g. N(7)—C(2) of compound 2) neighboring to the pyrazine ring possess significant double bond character by the lone nitrogen pair, which changes the charge distribution of the heterocyclic ring and leads to, in some cases, an increase in the aromaticity of the heterocyclic ring, thus stabilizing the ring system[15].
| Tab.2 Selected bond lengthsa of cyclourea nitramine compounds computed at B3LYP/6-31G** level |
| Tab.3 Dihedral angles of cyclourea nitramine compounds computed at B3LYP/6-31G** level |
However, it appears that two N—NO2 bonds with bond lengths of 1.517, 1.512 Å break in compound 7 after a formation of pyrazine N-oxide, which attributes to the obvious steric hindrance effect. It shows that the high instability of cyclic dinitrourea in the pyrazine N-oxide structure. The dihedral angles of the pyrazine ring are almost zero and six atoms can be considered as nearly coplanar. With nitro groups introduced, N—NO2 should rotate by some degrees from the pyrazine ring to avoid too large space steric effect. It is obvious that there are no intramolecular hydrogen bonds in cyclourea nitramine compounds of the pyrazine ring.
3.2 DensityIn the present study, single-point molecular volume calculations at B3LYP/6-31G** level were performed based on geometry optimized structures. The densities (ρ) of compounds 1-6 were calculated and listed in Table 4. It can be seen from Table 4 that the cyclourea nitramine compounds with different numbers or positions of nitro groups have different ρ values ranging from 1.84-2.03 g·cm-3. Compound 6 with four nitro groups has the largest density of 2.03 g·cm-3 among all nitro derivatives of symmetric pyrido-dicycloureas, while compound 1 with one nitro group has the smallest one, revealing that the density increases as the number of the nitro group increases. There is not much difference in the density for the compounds with the same amount of nitro groups in different positions(comparing compound 2 with compounds 3 and 4.Their detonation velocities increase with the increasing of density. Thus that compound 6 can be used as a novel potential candidate for HEDC when it is successfully synthesized.
| Tab.4 Predicted densities and detonation properties of cyclourea nitramine compounds |
The detonation velocity (D) and detonation pressure (p) of compounds are computed by Kamlet-Jacobs empirical equations on the basis of their theoretical densities (ρ) and calculated gas phase heats of formation. Table 4 shows the predicted detonation performances of nitro derivatives of symmetric pyrazino-dicycloureas.
It can be found that all nitro derivatives of symmetric pyrazino-dicycloureas have good detonation properties. Compound 6 has the highest D and p values among all cyclourea nitramine compounds. With the number of the nitro group increasing from one to four, ρ, Q, D, and p values of the corresponding compounds increase. The above predictions indicate that compound 6 is appearing to be a promising candidate comparable to RDX and HMX.
3.4 Heats of formation and oxygen balanceHeat of formation (HOF) reflects to the nature of substances and high positive HOF is usually required for an effective energetic material. The zero point energies (ZPE), thermal correction to enthalpy (HT), electronic energies and HOF calculated at B3LYP/6-31G** level for nitro derivatives of symmetric pyrazino-dicycloureas are listed in Table 5. The results show that all HOFs of cyclourea nitramine compounds vary from negative to positive values, revealing that the introduction of the nitro group is the main energy origin of this series. The HOFs of compounds increase when the number of the nitro group increases, which may be attributed to repulsion of the nitro groups. The greater the number of the nitro groups in the same ring (compare compound 2 with compounds 3 and 4) is, and the better molecular symmetry (compare compound 4 with compound 2) is, the greater the HOF is. the nitro group is an effective substituent for increasing HOF of the nitro derivatives of symmetric pyrazino-dicycloureas.
| Tab.5 Calculated electronic energies (E0), zero-point energies (ZPE), thermal correction to enthalpy (HT) and gas phase heats of formation (HOF) |
Oxygen balance (OB100) is another one of the most important criterion for selecting potential HEDC, and is a measure of how much oxygen is required for complete combustion of hydrogen to water and carbon to carbon dioxide. A positive or negative oxygen balance signifies that there is an excess of or a deficiency of oxygen in the complete combustion of compound. The oxygen balances are calculated using the formula (3), which can be used to rudely predict the impact sensitivities of the explosives[24].
| $ {\rm{O}}{{\rm{B}}_{100}} = 100(2{n_{\rm{O}}} - {n_{\rm{H}}} - 2{n_{\rm{C}}} - 2{n_{{\rm{COO}}}})/M $ | (3) |
Where nO, nH, and nC represent the numbers of O, H, and C atoms, respectively; nCOO is the number of COO-, and here nCOO=0 for the nitro derivatives of symmetric pyrazino-dicycloureas; M is the molecular weight. It is found from Table 4 that all title compounds have a negative oxygen balance, and when the amount of nitro group increases, the oxygen balance is close to zero, proving that the nitro group is a good substituent for improving oxygen balance in designing potential HEDC.
3.5 Thermal stabilityEnergies (a.u.) of frontier molecular orbital and their gaps of the nitro derivatives of symmetric pyrido-dicycloureas at B3LYP/6-31G** level are listed in Table 6. It can be seen from Table 6 that the ΔELUMO-HOMO values are different from different positions of substituted groups, the ΔELUMO-HOMO of compound 4 is the largest, while compound 2 is the smallest (except compound 7), indicating that the former is more stable than the latter.
| Tab.6 Energy of highest occupied molecular orbital (HOMO), energy of lowest unoccupied molecular orbital (LUMO) and energy gaps (ΔELUMO-HOMO) for cyclourea nitramine compounds containing pyridine ring |
In nitro compounds, N—NO2 bond is the weakest in the molecule and the rupture of this bond is the initial step in the decomposition or detonation. The property of N—NO2 bond, i.e., charge is used to show the relationship with the impact sensitivity of compounds, and may reflect the ability of —NO2 attracting electrons [25-26]. In the present study, the charge on nitro group (QNO2) is considered for its correlation to impact sensitivity.
| $ {Q_{{\rm{NO}}2}} = {Q_{\rm{N}}} + {Q_{{\rm{O}}1}} + {Q_{{\rm{O}}2}} $ | (4) |
The charge on nitro group (QNO2) is calculated by the sum of atomic charges on nitrogen (QN) and oxygen (QO1 and QO2) atoms in nitro group via. Eq.4. Computed QNO2 values of molecules are presented in Table 7. The higher the QNO2 is, the larger the impact insensitivity, and hence, QNO2 can be regarded as the criterion for estimating impact sensitivities. Compounds 2, 3 and 4 are isomers having two nitro groups attached to the pyrazine ring. Based on QNO2 values in Table 7, the stability of compounds 2, 3 and 4 decreases in the order of 4>3>2. This shows that compound 4 is more insensitive than compounds 2 and 3. This order is consistent with the order based on ΔELUMO-HOMO. An increase in the number of nitro groups (from one to four in compounds 1, 2, 5 and 6) increases the impact sensitivity(except compound 7). The above investigations provide important theoretic information for molecular design of novel high energetic density nitramine explosives containing pyrazine ring.
| Tab.7 Computed nitro group charge (—QNO2) of molecules 1-6 |
From the DFT calculations performed in this work, the main conclusions to be drawn are as follows.
(1) The number, positions, surrounding and symmetry of the nitro group within the molecule and the symmetry of the molecular structure are some principal factors affecting the thermal stabilities and detonation properties of compounds 1-6.
(2) With increasing of the number of the nitro group on five-membered rings within molecule, the values of heat of formation of compounds 1-6 increase.
(3) The calculated N(13)—N(19) and N7—N(22) bond lengths in compound 7 are1.517, 1.512 Å, respectively, which are the longest N—N bonds among all the N—N bonds in compounds 1-7, indicating that the two bonds will be broken first upon heating, so the structure of compound 7 is unstable.
(4) Using ΔELUMO-HOMO and QNO2 as criterions, the stability of compounds 2, 3 and 4 decreases in the order of 4>3>2.
(5) The calculated theoretical molecular density, heat of formation, detonation velocity and detonation pressure of compound 6 are 2.03g·cm-3, 265.63 kJ·mol-1, 9.08 km·s-1 and 39.22 GPa, respectively, revealing compound 6 might be a promising candidate for HEDC.
| [1] |
Pagoria P F, Mitchell A R, Jessop E S. Nitroureas Ⅱ.synthesis of bicyclic mono-and dinitrourea compounds[J]. Propellants, Explosives, Pyrotechnics, 1996, 21: 14-18. DOI:10.1002/(ISSN)1521-4087 |
| [2] |
Sikder A K, Bhokare G M, Sarwade D B, et al. Synthesis, characterization and thermal behaviour of 2, 4, 6, 8-tetranitro-2, 4, 6, 8-tetraazabicyclo[3.3.1]nonane-3, 7-dione(TNPDU) and one of its methylene analogues[J]. Propellants, Explosives, Pyrotechnics, 2001, 26: 63-68. DOI:10.1002/(ISSN)1521-4087 |
| [3] |
ZHOU Cheng, ZHOU Yan-shui, WANG Bo-zhou, et al. A novel synthetic route, crystal structure and thermal behavior for 1, 3, 5-trinitro-hexahydro-1, 3, 5-triazin-2(1H)-one (Keto-RDX)[J]. Chinese Journal Organic Chemistry, 2012, 32: 75-80. |
| [4] |
Sikder N, Bulakh N R, Sikder A K, et al. Synthesis, characterization and thermal studies of 2-oxo-1, 3, 5-trinitro-1, 3, 5-triazacyclohexane (Keto-RDX or K-6)[J]. Journal of Hazardous Materials, 2003, 96: 109-119. DOI:10.1016/S0304-3894(02)00169-3 |
| [5] |
MA Hai-xia, SONG Ji-rong, ZHAO Feng-qi, et al. Crystal structure, safety performance and density-functional theoretical investigation of 2, 6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105)[J]. Chinese Journal of Chemistry, 2008, 26(11): 1997-2002. DOI:10.1002/cjoc.v26:11 |
| [6] |
Tarver C M, Urtiew P A, Tran T D. Sensitivity of 2, 6-diamino-3, 5-dinitropyrazine-1-oxide[J]. Journal of Energetic Materials(Hanneng Cailiao), 2005, 23(3): 183-203. DOI:10.1080/07370650591001853 |
| [7] |
Gökçlnar E, Klapötke T M, Bellamy A J. Computational study on 2, 6-diamino-3, 5-dinitropyrazine and its 1-oxide and 1, 4-dioxide derivatives[J]. Journal of Molecular Structure: Theochem, 2010, 953(1): 18-23. |
| [8] |
Weese R K, Burnham A K, Turner H C, et al. Exploring the physical, chemical and thermal characteristics of a new potentially insensitive high explosive RX-55-AE-5[J]. Journal of Thermal Analysis and Calorimetry, 2007, 89(2): 465-473. DOI:10.1007/s10973-006-8163-4 |
| [9] |
XU Wen-zheng, AN Chong-wen, WANG Jing-yu, et al. Preparation and properties of an insensitive booster explosive based on LLM-105[J]. Propellants, Explosives, Pyrotechnics, 2013, 38(1): 136-141. DOI:10.1002/prep.v38.1 |
| [10] |
AN Chong-wei, LI He-qun, GENG Xiao-heng, et al. Preparation and properties of 2, 6-diamino-3, 5-dinitropyrazine-1-oxide based nanocomposites[J]. Propellants, Explosives, Pyrotechnics, 2013, 38(2): 172-175. DOI:10.1002/prep.v38.2 |
| [11] |
LIN Hin, ZHU Shun-guan, LI Hong-zhen, et al. Structure and detonation performance of a novel HMX/LLM-105 cocrystal explosive[J]. Journal of Physical Organic Chemistry, 2013, 26(11): 898-907. DOI:10.1002/poc.v26.11 |
| [12] |
Pagoria P F, Mitchell A, Schmidt R. Synthesis, scale-up and experimental testing of LLM-105[C]//Insensitive Munitions and Energetic Materials Technology Symposium, San Diego, CA. 1998.
|
| [13] |
Fried L E, Manaa M R, Pagoria P F, et al. Design and synthesis of energetic materials[J]. Annual Review of Materials Research, 2001, 31(1): 291-321. DOI:10.1146/annurev.matsci.31.1.291 |
| [14] |
ZHANG Chao-yang, SHU Yuan-jie, ZHAO Xiao-dong, et al. Computational investigation on HEDM of azoic and azoxy derivatives of DAF, FOX-7, TATB, ANPZ and LLM-105[J]. Journal of Molecular Structure: Theochem, 2005, 728(1): 129-134. |
| [15] |
Pagoria P F, Lee G S, Mitchell A R, et al. A review of energetic materials synthesis[J]. Thermochimica Acta, 2002, 384(1): 187-204. |
| [16] |
Boileau J, Emeury J M L, Kehren J P. Tetranitroglycoluril and method of preparation thereof: US, 4487938[P]. 1984-12-11.
|
| [17] |
Fischer J W, Hollins R A, Lowe-Ma C K, et al. Synthesis and characterization of 1, 2, 3, 4-cyclobutanetetranitramine derivatives[J]. The Journal of Organic Chemistry, 1996, 61: 9340-9343. DOI:10.1021/jo9613040 |
| [18] |
HU Rong-zu, LIANG Yan-jun, FANG Yin-gao, et al. The hydrolytic stability of some cyclourea compounds[J]. Journal of Thermal Analysis, 1996, 46: 1283-1289. DOI:10.1007/BF01979242 |
| [19] |
HU Rong-zu, LU Xing-sen, FANG Yin-gao. Thermal behaviour of 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaaza-tricyclo[7, 3, 0, 03, 7]dodecane-5, 11-dione[J]. Journal of Energetic Materials(Hanneng Cailiao), 1993, 11: 219-241. DOI:10.1080/07370659308227812 |
| [20] |
ZHANG Ji, XIAO He-ming, GONG Xue-dong. Theoretical studies on heats of formation for polynitrocubanes using the density functional theory B3LYP method and semiempirical MO methods[J]. Journal of Physical Organic Chemistry, 2001, 14(8): 583-588. DOI:10.1002/(ISSN)1099-1395 |
| [21] |
Chen Z X, Xiao J M, Xiao H M, et al. Studies on heats of formation for tetrazole derivatives with density functional theory B3LYP method[J]. The Journal of Physical Chemistry A, 1999, 103(40): 8062-8066. DOI:10.1021/jp9903209 |
| [22] |
Kamlet M J, Jacobs S J. Chemistry of detonations. I. A simple method for calculating detonation properties of C—H—N—O explosives[J]. The Journal of Chemical Physics, 1968, 48: 23-25. DOI:10.1063/1.1667908 |
| [23] |
WANG Gui-xiang, GONG Xue-dong, LIU Yan, et al. A theoretical investigation on the structures, densities, detonation properties and pyrolysis mechanism of the nitro derivatives of toluenes[J]. Journal of Hazardous Materials, 2010, 177: 703-710. DOI:10.1016/j.jhazmat.2009.12.088 |
| [24] |
Kamlet M J, Adolph H G. The relationship of impact sensitivity with structure of organic high explosives[J]. Propellants, Explosives, Pyrotechnics, 1979(4): 30-34. |
| [25] |
ZHANG Chao-yang. Review of the establishment of nitro group charge method and its applications[J]. Journal of Hazardous Materials, 2009, 161: 21-29. DOI:10.1016/j.jhazmat.2008.04.001 |
| [26] |
ZHANG Chao-yang, SHU Yuan-jie, HUANG Yi-gang, et al. Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds[J]. The Journal of Physical Chemistry B, 2005, 109: 8978-8982. DOI:10.1021/jp0512309 |

The molecular geometries and electronic structures of nitro derivatives of symmetric pyrazino-dicycloureas were obtained at the B3LYP/6-31G** level.Their theoretical molecular density (ρ) and heat of formation (HOF) were computed by quantum chemical method and detonation velocity (D) and detonation pressure (p) were estimated using Kamlet-Jacobs equations.




