2. Shaanxi Institute of Technology, Xi′an 710300, China
2. 陕西国防工业职业技术学院,陕西 西安 710300
Five-membered nitrogen-containing heterocycles, with high thermal stability and positive heats of formation, have been recognized as the ideal precursors of high energy density materials (HEDM) [1-2].Their high heats of formation is due to the large number of N—N and C—N bonds [3-4]. A number of five-membered heterocycle-based energetic compounds were reported as potential materials for military and space applications[5-7]. Of these, furazan ring served as an efficient build-block. The combination of furazan ring with energetic substituents, such as amino (—NH2)[8], nitro (—NO2)[9], azide (—N3)[10] and nitramine (—NHNO2)[11] have been widely investigated.
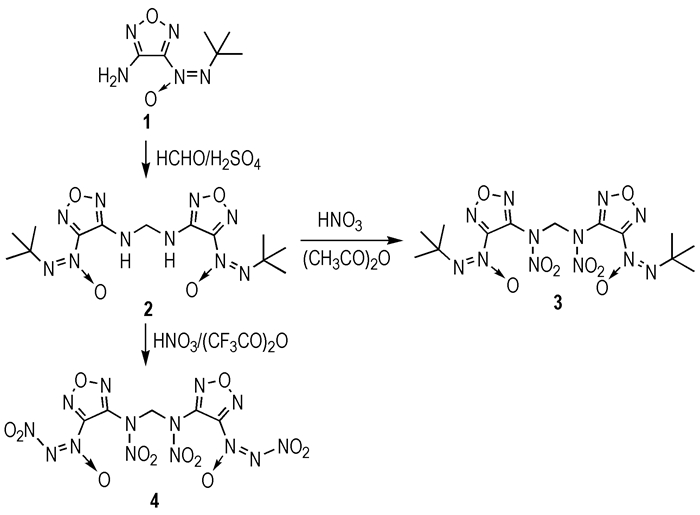
As an unconventional explosophoric group, nitro-NNO-azoxy was first reported by Churakov et al [12]. After that, bis-3,3′-(nitro-NNO-azoxy)-difurazanyl ether [13] and bis-3,3′-(nitro-NNO-azoxy)-4,4′-azofurazan [14] was synthesized by Sheremetev and our group respectively, which are both of high crystal density and excellent detonation properties. In continuation of our study on the furazan-functionalized energetic material, a novel energetic compound, namely N, N′-dinitro-N, N′-bis [3-(nitro-NNO-azoxy)furazan-4-yl] methylenediamine (4), was designed and synthesized firstly from 3-amino-4-(t-Bu-NNO-azoxy) furazan (1) in this study. Its structure was confirmed by IR, NMR (1H NMR and 13C NMR), elemental analysis and mass spectrometry. The nitration reaction of N, N′-bis [3-(t-Bu-NNO-azoxy) furazan-4-yl] methylenediamine (2) was also studied, and two nitration derivatives were obtained at different reaction conditions, which were not reported in the literature. Furthermore, the detonation performance of 4 was also calculated.
2 Experimental 2.1 Synthetic route
|
Scheme1 |
1H NMR and 13C NMR were carried out on the Bruker AV500 NMR spectrometer. Infrared spectra were tested with KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000~400 cm-1. Elemental analyses (C, H and N) were performed on a VARI-El-3 elemental analyzer. Mass spectra were obtained on GCMS-QP2010. 3-amino-4-(t-Bu-NNO-azoxy)furazan [14] and 100% nitric acid were prepared in our laboratory.
2.3 Synthesis of 237% formaldehyde (0.86 g, 10.6 mmol) was added to a suspension of 1 (3.93 g, 21.0 mmol) in 50% sulfuric acid (100 g), then the mixture was stirred at 30 ℃ overnight. The yellow precipitate was filtered, washed with water and dried to obtain 3.68 g of solid in a yield of 92.2%. 1H NMR (DMSO-d6, 500 MHz): 1.43 (s, 18H), 4.87 (t, 2H), 7.14 (t, 2H); 13C NMR (DMSO-d6, 125 MHz): 151.30, 150.14, 60.07, 53.32, 25.07; IR (KBr, ν/cm-1): 3472, 3411, 3387, 2972, 1623, 1610, 1537, 1454, 1379, 1301, 1181, 1119, 910, 861 cm-1; Anal. Calcd. (%) for C13H22N10O4: C 40.83, H 5.80, N 36.63; Found (%): C 40.85, H 5.74, N 36.72.
2.4 Synthesis of 32 (0.38g, 1 mmol) was added to a mixture of acetic anhydride (2.5 mL), CCl4 (4 mL) and anhydrous nitric acid (1.0 mL, 23.5 mmol) at 10℃, then the mixture was stirred at room temperature for 6 h. The reaction mixture was poured to ice-water (30 mL), then extracted five times with CH2Cl2 (15 mL). The extraction were washed with water and dried over magnesium sulfate, filtered and the solvent was removed to give 0.39 yellow solid in a yield of 82.6%. 1H NMR (Acetone-d6, 500 MHz): 1.43 (s, 18H), 6.64 (s, 2H); 13C NMR(Acetone-d6, 125 MHz): 156.70, 147.36, 67.50, 61.78, 25.18; IR (KBr, ν/cm-1): 2971, 1753, 1606, 1478, 1452, 1300, 1283, 1125, 904, 859 cm-1; Anal. Calcd. (%) for C13H20N12O8: C 33.05, H 4.27, N 35.58; Found (%): C 32.95, H 4.30, N 35.72.
2.5 Synthesis of 42 (0.38g, 1mmol) was added to a mixture of trifluoroacetic anhydride (13.5 mL) and anhydrous nitric acid (3.4 mL, 0.08 mol) at room temperature, then the mixture was stirred at 37 ℃ for 5 h. The reaction mixture was poured to ice-water (50 mL), then extracted five times with CH2Cl2(15 mL). The extraction were washed with water and dried over magnesium sulfate, filtered and the solvent was removed to give yellow oil. The title compound(4) was purified by column chromatography on silica gel using a mixture of petroleum ether and ethyl acetate (Rf=0.4, 3:1, V/V) as an eluent, affording 0.3 g of yellow oil in a yield of 66.7%. 1H NMR(Acetone-d6, 500 MHz): 6.92 (s, 2H); 13C NMR (Acetone-d6, 125 MHz): 153.67, 146.94, 66.73; IR (KBr, ν/cm-1):3041, 2906, 1784, 1636, 1610, 1556, 1495, 1369, 1283, 1220, 1172, 1082, 1023, 946, 835 cm-1; MS (ESI-) m/z (%): 512 (M+NO3-); Anal. Calcd. (%) for C5H2N14O12: C 13.34, H 0.45, N 43.56; Found (%): 13.30, H 0.44, N 43.50.
2.6 Determination of the Crystal Structure of 2Block-like single crystals suitable for X-ray diffraction studies were obtained after 15 days by slow evaporation from a solution of compound 2 in CHCl3 at room temperature. A yellow crystal of compound 2 with dimensions of 0.15 mm×0.15 mm×0.10 mm was chosen for X-ray diffraction analysis performed on Bruker SMART APE II CCD X-ray diffractometer with a MoKα radiation (λ=0.71073Å) by using a φ-ω scan mode at 296 (2) K. In the range of 2.10°≤θ≤28.36°, a total of 5991 reflections were collected including 4424 unique ones (Rint =0.0186). The structure was solved by direct methods using SHELXS program of the SHELXL-97 package and refined with SHELXL package [15]. The final refinement was performed by full-matrix least-squares method with anisotropic thermal parameters on F2 for the non-hydrogen atoms.
The crystal belongs to the triclinic system, space group P-1 with a= 9.918(4)Å, b= 10.748(4)Å, c=10.924(4)Å, α =98.170(5)°, β =113.345(6)°, γ =108.484(6)°, Mr=382.41, V=964.8(6)Å3, Z=2, Dc=1.316 g·cm-3, F(000) =404, μ=0.101 mm-1, R=0.0476 and wR=0.1053. Crystallographic data for the structure reported here have been deposited with the Cambridge Crystallographic Data Centre (Deposition No. CCDC-961282).
3 Results and Discussion 3.1 Synthesis1 was synthesized according to literature [14], and 2 was synthesized using 1 and formaldehyde by the aldehyde-amine condensation that widely used in constructing the backbone of HEDMs. The reaction progress was monitored by Thin-Layer Liquid Chromatography(TLC). With 10% aq. H2SO4 used, the reaction mixture contained the starting material 1. While with 20%~30% aq. H2SO4, the reaction mixture contained 1 and small amount of 2. The optimal conditions for the preparation of 2 are stirring the starting components in 50% aq. H2SO4 at room temperature, and its yield is 92.2%.
Treating 2 with different nitrolysis agent may genarate different products. When 100% HNO3-Ac2O mixture used, the dinitramine compound 3 was obtained in a yield of 82.6%. When a stronger nitrolysis agent 100% HNO3- (CF3CO)2O, TLC results show that 3 was appeared firstly and then transformed into 4.
3 and 4 were well characterized by IR, 13C NMR, 1H NMR, elemental analysis. In the IR spectra, 3 and 4 both have several main absorption bands which can attributed to the furazan ring and nitro group respectively. In the 13C NMR spectrum, the resonances of the furazan ring and methylene (—CH2—) carbon was more downfield for 3 at 156.70, 147.36, 67.50, and 153.67, 146.94, 66.73 for 4 respectively. Furthermore, the M+NO3- peak of 4 at 512 m/z was detected and the formation of 4 was confirmed.
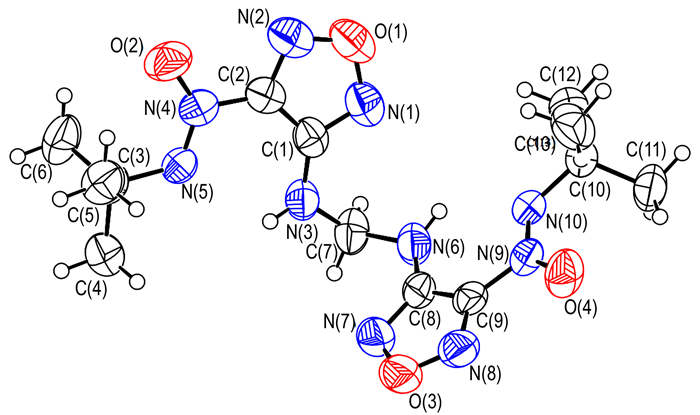
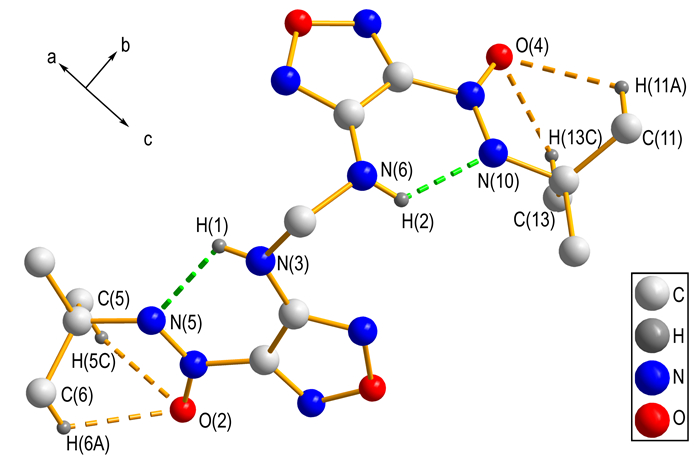
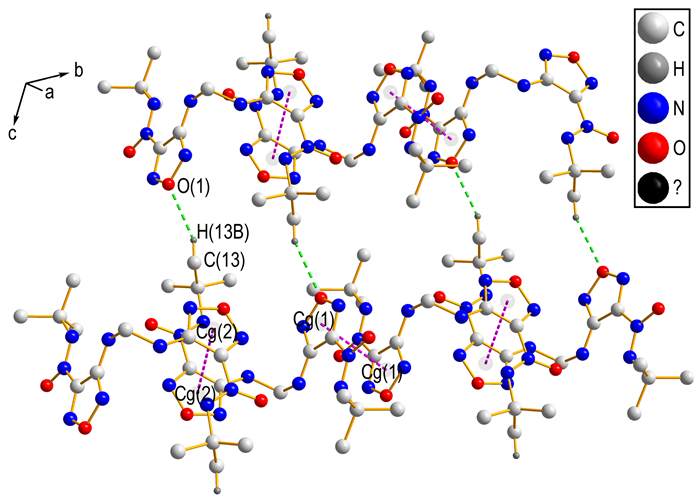
3.2 Crystal StructureIn order to elucidate the structure of backbone in the title compound, the single crystal of key intermediate 2 was obtained and studied. A perspective view of compound 2, showing the atomic numbering scheme, is given in Fig. 1. In each molecule, the dihedral angle between furazan planes defined by O(1)\N(1)\C(1)C(2)N(2) and O(3)\N(7)\C(8)C(9)N(8), is about 78.31 (2)°. As shown in Fig. 2, intramolecular hydrogen bonds N—H…N and C—H…O forming S (6) motif produce six-membered ring and stabilize the molecular structure. Intermolecular hydrogen bonding C(13)—H(13)B…O(1) between alkyl carbon and furazan oxygen links neighboring molecule into dimer with a motif of R22(26). Furthermore, in Fig. 3, stronger π…π stacking interactions among adjacent furazan rings further stabilizes the crystal structure. With the help of above mentioned hydrogen bond and π…π stacking interactions, the title compound(4) was obtained with an infinite 2D sheet-like supermolecular structure. Hydrogen atoms in Fig. 2 and Fig. 3, except those involved in hydrogen bonds, have been omitted for clarity.
|
Fig.1 ORTEP view of the crystal structure with thermal ellipsoids drawn at the 30% probability for title compound |
|
Fig.2 Diagram of the intramolecular hydrogen bonds |
|
Fig.3 Diagram of the intermolecular hydrogen bonds and π …π interactions |
The structure of 4 was optimized and its density and enthalpy of formation were calculated by Gaussian 09[16]. The explosive parameters were obtained by VLW equation of state[17] using density and enthalpy of formation as basic data. Results show that the density is 1.94 g·cm-3, enthalpy of formation 1007.67 kJ·mol-1, detonation velocity 9502.52 m·s -1and C-J pressure 41.79 GPa. The calculated detonation parameters are comparable to CL-20 (9406 m·s -1, 44.6 GPa)[18].
4 ConclusionsIn conclusion, a novel furazan compound (4) was synthesized and characterized. The crystal structure of 2 was studied for confirming the backbone of title compound (4) and it forms an infinite 2D sheet-like supermolecular structure. Furthermore, the calculations indicate the detonation properties of 4 are comparable to CL-20. Results show that the density 1.94 g·cm-3, enthalpy of formation 1007.67 kJ·mol-1, detonation velocity 9502.52 m·s -1and C-J pressure 41.79 GPa.
| [1] |
Dippold A A, Klapötke T M. A study of dinitro-bis-1, 2, 4-triazole-1, 1′-diol and derivatives: design of high-performance insensitive energetic materials by the Introduction of N-oxides[J]. J Am Chem Soc, 2013, 135: 9931-9938. DOI:10.1021/ja404164j |
| [2] |
Zhai L J, Wang B Z, Huo H, et al. Synthesis, disproportionation and hydrolysis reactions of 3-Amino-4-hydroxyaminoximinofurazan[J]. Chin J Org Chem, 2013, 33(8): 1755-1761. DOI:10.6023/cjoc201302001 |
| [3] |
Gao H X, Shreeve J M. Azole-based energetic salts[J]. Chem Rev, 2011, 111: 7377-7436. DOI:10.1021/cr200039c |
| [4] |
Kerth J, Lobbecke S. Synthesis and characterization of 3, 3′-azobis(6-amino-1, 2, 4, 5-tetrazine) DAAT-a new promising nitrogen-rich compound[J]. Propell Explos Pyrot, 2002, 27: 111-118. DOI:10.1002/1521-4087(200206)27:3<111::AID-PREP111>3.0.CO;2-O |
| [5] |
Fischer N, Fischer D, Klapötke T M, et al. Pushing the limits of energetic materials-the synthesis and characterization of dihydroxylammonium 5, 5′-bistetrazole-1, 1′-diolate[J]. J Mater Chem, 2012, 22: 20418-20422. DOI:10.1039/c2jm33646d |
| [6] |
Li H, Wang B Z, Lai W P, et al. Synthesis of 3, 4-dinitrofuroxan[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2013, 21(3): 396-397. |
| [7] |
Thottempudi V, Gao H X, Shreeve J M. Trinitromethyl-substituted 5-nitro-or 3-azo-1, 2, 4-triazoles: synthesis, characterization, and energetic properties[J]. J Am Chem Soc, 2011, 133: 6464-6471. DOI:10.1021/ja2013455 |
| [8] |
Chavez D, Hill L, Hiskey M, et al. Preparation and explosive properties of azo-and azoxy furazans[J]. J Energ Mater, 2000, 18: 219-236. DOI:10.1080/07370650008216121 |
| [9] |
Zhou Y S, Wang B Z, Li J K, et al. Study on synthesis, characterization and properties of 3, 4-bis (4′-nitrofurazano-3′-yl)furoxan[J]. Acta Chimica Sinica, 2011, 69(14): 1673-1680. |
| [10] |
Zhou Y S, Wang B Z, Zhou C, et al. Synthesis, characterization and crystal structure study on 3, 4-bis (4′-azidofurazano-3′-yl)furoxan[J]. Chin J Org Chem, 2010, 30(7): 1044-1050. |
| [11] |
Philip F P, Gregory S L, Alexander R M, et al. A review of energetic materials synthesis[J]. Thermochimi Acta, 2002, 384: 187-204. DOI:10.1016/S0040-6031(01)00805-X |
| [12] |
Churakov A M, Loffe S L, Tartakovskii V A. Synthesis of l-aryl-2-nitrodiazene 1-N-oxides[J]. Mendeleev Commun, 1996, 27: 20-22. |
| [13] |
Sheremetev A B, Semenov S E, Kuzmin V S, et al. Synthesis and x-ray crystal structure of bis-3, 3′-(nitro-NNO-azoxy)-difurazanyl ether[J]. Chem Eur J, 1998, 4: 1023-1026. DOI:10.1002/(ISSN)1521-3765 |
| [14] |
Li H, Wang B Z, Li X Z, et al. Synthesis and crystal Structure of bis-3, 3′-(nitro-NNO-azoxy)-4, 4′-azofurazan[J]. Bull Korean Chem Soc, 2013, 34(2): 687-689. |
| [15] |
Sheldrick G M. SHELX97, Programs for crystal structure analysis. Institut für Anorganische Chemie der Universität, Göttingen, Germany, 1998.
|
| [16] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09. Gaussian, Inc., Wallingford CT, 2009.
|
| [17] |
Wu X, Long X P, He B, et al. VLW equation of state of detonation products[J]. Sci China, Ser B, 2009, 52(5): 605-608. DOI:10.1007/s11426-009-0094-z |
| [18] |
Song J H, Zhou Z M, Dong X, et al. Super-high-energy materials based on bis (2, 2-dinitroethyl)nitramine[J]. J Mater Chem, 2012, 22: 3201-3209. DOI:10.1039/c2jm15310f |