2. School of Chemical Engineering, Nanjing University of Science & Technology, Nanjing 210094, China;
3. School of Pharmacy, China Pharmaceutical University, Nanjing 210009, China
2. 南京理工大学化工学院, 江苏 南京 210094;
3. 中国药科大学药学院, 江苏 南京 210009
Materials that can store and release large amounts of chemical energy on demand have a wide variety of applications. These materials must possess properties that allow them to be handled safely. Recently, the development of new energetic materials, focus on a class of so-called "high-nitrogen" compounds whose energy is derived from their very high positive heat of formation[1-3]. Thus, superior energetic performances may also potentially be achieved by incorporation of azo-, azole-, azine-based building blocks[4-7].
Many papers reveal that pyridine-based compounds have captured a major role in energetic material, and the potential uses of nitropyridine derivatives have been reported for the synthesis of novel insensitive explosives[8-11]. These compounds take the inherent stability of the aromatic heterocyclic ring system and combine it with the insensitivity and stability associated with alternating amino and nitro groups.
Pursuing our interests in extending the chemistry of energetic pyridines, and on combination azo-functionality with pyridine analogues, we initiated our work to develop azo-bridged pyridine derivatives by using 2-chloro-4aminopyridine (1) as a primary material, and this is a new synthetic route to provide pyridine-based energetic materials bearing amino, nitro and azo groups.
2 Experimental 2.1 Materials and InstrumentsThe starting materials used in the present study were of AR grade. Column chromatographic purifications were performed on silica gel with 100-200 mesh. Melting point was measured on an X-4 melting point apparatus without correction. 1H NMR (500 MHz) and 13C NMR (125 MHz) were recorded on a Bruker Advance Spectrometer (TMS as an internal standard). High-resolution mass spectra were recorded on a Finnigan TSQ Quantum ultra AM mass spectrometer. Elemental analysis was carried out on a Perkin-Elmer instrument.
2.2 Synthetic Routes
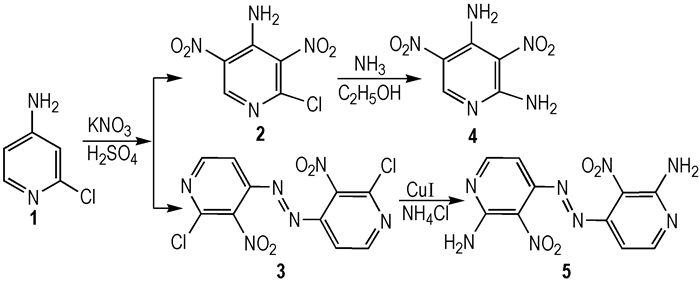
|
Scheme1 |
Compound 1 (3.20 g, 1, 25.00 mmol) was dissolved in 75 mL of concentrated sulfuric acid at room temperature, and potassium nitrate (10.10 g, 100.00 mmol) was added in portions with vigorous stirring. After the solution was clear, the reaction mixture was heated to 60 ℃ for 4 h and allowed to cool to room temperature. After pouring over ice, the mixture was filtered, washed with water to give a crude product. The light brown solid was dissolved in 50 mL ethyl acetate, and then filtered. The filtrate was dried over Na2SO4 and evaporated under reduced pressure to give light brown solid substance 2 of 3.27 g with yield 60%. m.p. 106-109 ℃; 1H NMR (DMSO-d6, 500 MHz) δ : 8.95 (t, 1H), 8.50 (s, 2H); 13C NMR (DMSO-d6, 125 MHz) δ : 148.00, 144.70, 142.51, 133.43, 130.25; Anal. calcd for C5H3ClN4O4:C 27.48, H 1.38, N 25.64; found: C 27.53, H 1.45, N 25.53; MS (ESI) m/z: 216.91:218.89=3:1(M-H). The filter cake was dissolved in 30 mL methanol, and then filtered to give red solid substance 3 of 0.98 g with yield 23%. m.p. 224-226 ℃; 1H NMR (CDCl3, 500 MHz)δ: 8.77 (d, J=5.3 Hz, 2H), 7.52 (d, J=5.3 Hz, 2H); 13C NMR (DMSO-d6, 125 MHz)δ : 151.11, 147.48, 142.83, 108.52; Anal. calcd for C10H4Cl2N6O4: C 35.01, H 1.18, N 24.50; found: C 35.04, H 1.16, N 24.53.
2.4 Synthesis of 2, 4-Diamine-3, 5-dinitropyridine (4)To a stirred solution of 2 (0.30 g, 1.38 mmol) in 25 mL of methanol was added dry ammonia for about 30 min at 0 ℃, and another 3 h at room temperature. The precipitate was filtered, washed with water and dried to give gray solid substance 4 of 0.27 g with yield 98.6%. m.p. >270 ℃; 1H NMR (DMSO-d6, 500 MHz)δ: 9.47 (s, 1H), 9.33 (s, 1H), 9.10 (s, 1H), 9.03 (s, 1H), 8.91 (s, 1H); 13C NMR (DMSO-d6, 125 MHz)δ: 157.34, 153.13, 147.40, 121.76, 113.24; Anal. calcd for C5H5N5O4: C 30.16, H 2.53, N 35.17; found C 30.12, H 2.55, N 35.23; MS (ESI) m/z: 197.99 (M-H).
2.5 Synthesis of (E)-1, 2-bis(2-amino-3-nitro-pyridin-4-yl)diazene(5)To a stirred solution of 3 (2.5 g, 7.31 mmol) in 10 mL of N, N-dimethylformamide (DMF) was added ammonium chloride (1.12 g, 20.94 mmol) in portions, and then a catalytic amount of cuprous iodide. The resulting mixture was then heated to 60 ℃ for 2.5 h, at which time complete consumption of the starting material was observed by thin layer chromatography. The reaction mixture was then poured over ice, and filtered, washed with water, and dried on air. The crude material was then purified via a silica gel column using EtOAc/PE as eluting solvent to give white solid substance 5 of 1.73 g with yield 78%. m.p. 154-156 ℃; 1H NMR (DMSO-d6, 500 MHz)δ: 8.96 (d, J=5.25 Hz, 2H), 8.61 (d, J=5.25 Hz, 2H), 7.89 (s, 1H), 7.42(s, 1H); Anal. calcd for C10H8N8O4: C 39.48, H 2.65, N 36.83; found C 39.45, H 2.63, N 36.86.
Single crystals suitable for X-ray diffraction were obtained from slow evaporation of solvent CHCl3 at room temperature.
2.6 Crystal Structure DeterminationRed block single crystal of 3 (0.30 mm×0.20 mm×0.10 mm) was collected by a Nonius cad4 detector equipped with a graphite-monochromatic Mo Kα radiation (λ=0.71073 Å) using a ω/2θ scan mode at 293(2) K. A total of 1306 reflections were collected in the range of 2.70°≤θ≤25.39°, among which 1234 (Rint=0.0355) was independent and 962 was observed with I>2σ(I). The structure was solved by direct methods and expanded by difference Fourier techniques with SHELXL-97 program. All the non-hydrogen atoms were anisotropically refined, and the hydrogen atoms were added according to the theoretical models. The full-matrix least-squares refinement gave R1=0.0528, wR2=0.1419 (w=1/[σ2 (Fo2)+(0.1000P)2+0.1800P)], where P=(Fo2+2Fc2)/3), S=1.004, (Δρ)max=0.247, (Δρ)min=-0.350 e/ Å3, (Δ/σ)max= 0.000 and (Δ/σ)mean=0.000.
3 Results and Discussion 3.1 Synthesis DescriptionWhen compound 1 was reacted with concentrated sulfuric acid and potassium nitrate at 60 ℃, compound 2 (major) and azo compound (3, minor, not identified until X-ray quality crystals grow up) were isolated. Extending the reaction time or raising the reaction temperature did not change compound 3 into the major product. To verify the formation of compound 3, it was shown that when compound 2 was isolated and then reacted with concentrated sulfuric acid and potassium nitrate at the same condition, and no new compound was found as the possible product. The result showed that nitration and diazo coupling reaction of compound 1 involved a parallel reaction, and once —NH2 in the pyridine ring formed strong hydrogen bond with nitro groups at ortho positions, reactions could′t occur even in harsh conditions. So it was likely that compound 1 underwent the oxidation and nitration processes successively to generate 3 (Scheme 2).
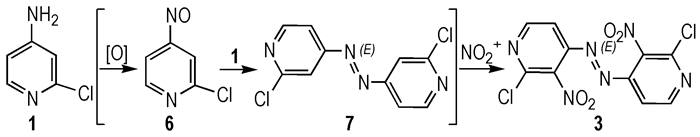
|
Scheme2 |
Polyamino and nitro pyridines play an important role in energetic heterocyclic compounds which have been investigated as potential new insensitive explosives. When compound 2 reacted with ammonia in ethanol, compound 4 was obtained in a high yield smoothly. However, subsequent amination reaction of compound 3 with ammonia or ammonium chloride (NH4Cl) failed, but succeeded with ammonium chloride and a catalytic amount of cuprous iodide to give compound 5 as a red solid. The result showed that the reaction might take place due to Cu-catalyzed C—N cross-coupling reaction mechanism, but not according to the more classical addition-elimination mechanism based on too many conjugated double bonds[12].
3.2 Structural DescriptionThe molecular structure and perspective view of the crystal packing in a unit cell of compound 3 are shown in Figs. 1 and 2, respectively. The selected bond lengths, bond angles and torsion angles are shown in Tables 1 and 2, respectively. Crystal data and structure refinement of compound 3 (CCDC: 956288) are shown in Table 3.
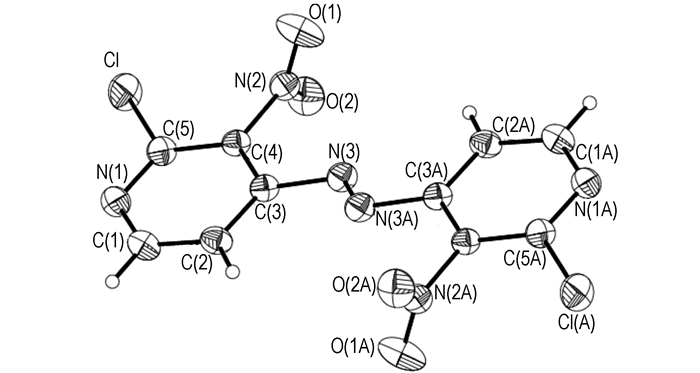
|
Fig.1 Molecular structure of compound 3 |
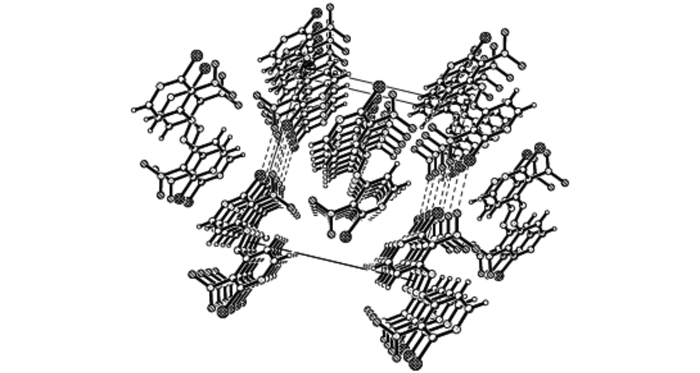
|
Fig.2 Packing of the molecule 3 in the crystal lattice |
| Tab.1 Selected bond lengths and bond angles for compound 3 |
| Tab.2 Selected torsion angles for compound 3 |
| Tab.3 Crystal data and structure refinement for compound 3 |
Compound 3 crystals in a trans-form, indicating that it may have lower energy than its cis-form. Cl—C(5) and C(4)—N(2) bond of the nitro group are nearly coplanar with the pyridine ring and twisted out of the pyridine plane by 0.7° and -179.7°, respectively. However, the azo-bond of N(3)—N(3A) is not coplanar with the pyridine ring, which twists out of it by -15.6°.
3.3 Theoretical StudiesTheoretical calculations were carried out by the Gaussian 09 program suite[13]. The geometry optimization of the molecular structure and frequency analyses were carried out by using the B3LYP functional with the 6-31G** basis set[14]. The optimized structure was characterized to be true local energy minima on the potential-energy surface without imaginary frequencies.
Detonation velocity (D) and detonation pressure (p) are the most important targets of scaling the detonation characteristics of energetic materials. For CHNO type explosives, the Kamlet-Jacobs empirical equations[15-16] was used to determine these parameters.
Kamlet-Jacobs empirical equations:
| $ D = 1.01{({N}{{M}^{0.5}}{{Q}^{0.5}})^{0.5}}(1 + 1.30{\rho _{\rm{o}}}) $ | (1) |
| $ p = {\rm{1}}{\rm{.55}}\rho _{\rm{o}}^2N{M^{0.5}}{Q^{0.5}} $ | (2) |
Where D is detonation velocity in km·s-1, p is detonation pressure in GPa, N is moles of gaseous detonation products per gram of explosives, M is the average molecular weights of gaseous products, Q is the energy of explosion in J·g-1 of explosive and ρo is the crystal density in g·cm-3.N, M and Q are determined according to the largest exothermic principle, i.e., for the CHNO explosives, all the N atom converts into N2, the O atom forms H2O with H atom first and the remainder forms CO2 with C atom. The remainder of C atom will exist in solid state if O atom does not satisfy full oxidation of C atom. The remainder of O atom will exist in O2 if O atom is superfluous.
The calculated results in Table 4 indicate that compound 4 has a similar oxygen balance and detonation performance comparing to TNT[17], but is worse than hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (RDX)[18], and compound 5 is less competitive. However, as a new azo-bridged pyridine derivative, compound 5 has a high value of density, due to the introduction of the alternate amino and nitro group in the pyridine ring. Thus, the continuous work will focus on how to improve its derivatives′detonation performance through different synthetic routes.
| Tab.4 Detonation performance of compounds 4 and 5 and comparison with TNT and RDX |
(1) The present investigation demonstrates the synthesis of two new energetic polyamino and nitro pyridine derivatives 5 and 4 with a total yield of 69% and 18%, respectively.
(2) Compound 3 is first synthesized and structurally determined by single crystal X-ray diffraction. It is monoclinic with space group P21/N.
(3) The detonation velocity and detonation pressure obtained by theoretical calculation are 7.27 km·s-1 and 23.13 GPa for compound 4 and 6.16 km·s-1 and 16.11 GPa for compound 5.
| [1] |
Dippold A A, Izsak D, Klapötke T M. A study of 5-(1, 2, 4-triazol-C-yl) tetrazolo-1-ols: combining the benefits of different heterocycles for the design of energetic materials[J]. Chemistry-A European Journal, 2013, 19: 12042-12051. DOI:10.1002/chem.201301339 |
| [2] |
Zhang Q, Zhang J, Parrish D A, et al. Energetic N-trinitroethyl-substituted mono-, di-, and triaminotetrazoles[J]. Chemistry-A European Journal, 2013, 19: 11000-11006. DOI:10.1002/chem.201300994 |
| [3] |
Fischer D, Klapötke T M, Piercey D G, et al. Synthesis of 5-aminotetrazole-1N-oxide and its azo derivative: a key step in the development of new energetic materials[J]. Chemistry-A European Journal, 2013, 19: 4602-4613. DOI:10.1002/chem.201203493 |
| [4] |
Huynh M H V, Hiskey M A, Hartline E L, et al. Polyazido high-nitrogen compounds: hydrazo-and azo-1, 3, 5-triazine[J]. Angewandte Chemie International Edition, 2004, 43: 4924-4928. DOI:10.1002/(ISSN)1521-3773 |
| [5] |
Vo T T, Zhang J, Parrish D A, et al. New roles for 1, 1-diamino-2, 2-dinitroethene (FOX-7): halogenated FOX-7 and azo-bis (diahaloFOX) as energetic materials and oxidizers[J]. Journal of the American Chemical Society, 2013, 135: 11787-11790. DOI:10.1021/ja406629g |
| [6] |
Thottempudi V, Shreeve J M. Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1H-1, 2, 4-triazole and 5, 5'-bis(trinitromethyl)-3, 3'-azo-1H-1, 2, 4-triazole[J]. Journal of the American Chemical Society, 2011, 133: 19982-19992. DOI:10.1021/ja208990z |
| [7] |
Qi C, Li S H, Li Y C, et al. Synthesis and promising properties of a new family of high-nitrogen compounds: polyazido-and polyamino-substituted N, N'-azo-1, 2, 4-triazoles[J]. Chemistry-A European Journal, 2012, 18: 16562-16570. DOI:10.1002/chem.201202428 |
| [8] |
Ghule V D, Srinivas D, Muralidharan K. Energetic monoanionic salts of 3, 5-dinitropyridin-2-ol[J]. Asian Journal of Organic Chemistry, 2013, 2: 662-668. DOI:10.1002/ajoc.201300079 |
| [9] |
Liu J J, Liu Z L, Cheng J, et al. Synthesis, crystal structure and properties of energetic complexes constructed from transition metal cations (Fe and Co) and ANPyO[J]. RSC Advances, 2013, 3: 2917-2923. DOI:10.1039/c2ra22839d |
| [10] |
MA Cong-ming, WANG Yong-bin, LIU Zu-liang, et al. Synthesis and performance of 7-amino-6-nitro-[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(4): 572-575. |
| [11] |
CHENG Jian, YAO Qi-zheng, LIU Zu-liang. Synthesis of 2, 6-diamino-3, 5-dinitropyridine-1-oxide[J]. Chinese Journal of Energetic Materials, 2009, 17(2): 166-168. |
| [12] |
Hou K H, Liu Z L. Synthesis and characterization of some N-aryl-4-nitroimidazoles as potential insensitive energy materials[J]. Chinese Journal of Chemistry, 2013, 31: 243-246. DOI:10.1002/cjoc.v31.2 |
| [13] |
Frisch M J, Trucks G W, Schlegel H B, et al, Gaussian 09[CP], Wallingford CT, 2009.
|
| [14] |
Chen Z X, Xiao J M, Xiao H M, et al. Studies on heats of formation for tetrazole derivatives with density functional theory B3LYP method[J]. The Journal of Physical Chemistry A, 1999, 103(40): 8062-8066. DOI:10.1021/jp9903209 |
| [15] |
Kamlet M J, Jacobs S J. Chemistry of Detonations. I. A simple method for calculating detonation properties of C—H—N—O explosives[J]. The Journal of Chemical Physics, 1968, 48(1): 23-35. DOI:10.1063/1.1667908 |
| [16] |
Kamlet M J, Ablard J E. Chemistry of Detonations. Ⅱ. Buffered Equilibria[J]. The Journal of Chemical Physics, 1968, 48(1): 36-41. DOI:10.1063/1.1667930 |
| [17] |
Wang G X. Theoretical study on the structure and performance of high energetic compounds[D]. Nanjing: Nanjing University of Science and Technology, 2008.
|
| [18] |
Qiu Ling, Xiao Heming, Gong Xuedong, et al. Theoretical studies on the structures, thermodynamic properties, detonation properties, and pyrolysis mechanisms of spiro nitramines[J]. The Journal of Physical Chemistry A, 2006, 110(10): 3797-3807. DOI:10.1021/jp054169g |
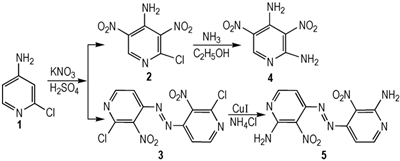
A newazo-bridged pyridine derivative (E)-1, 2-bis(2-chloro-3-nitropyridin-4-yl) diazene (3) and 2-chloro-4-amine-3, 5-dinitropyridine (2) were synthesized through a direct nitration reaction using 2-chloro-4-aminopyridine(1) as a primary material, followed by a simple amination reaction to give (E)-1, 2-bis(2-amino-3-nitro-pyridin-4-yl)diazene(5) and 2, 4-diamino-3, 5-dinitropyridine(4).




