2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China
2. 西安近代化学研究所, 陕西 西安 710065
1, 1-Diamino-2, 2-dinitroethylene (FOX-7) is a novel high-energy material with high thermal stability and low sensitivity, which has a same insensitivity to TATB and a similar energy density with RDX and HMX[1]. When first synthesized in 1998[1], FOX-7 received much attention and was considered as the main component potentially in insensitive ammunitions and solid propellants. Many researches have been carried out about the synthesis, mechanism, molecule structure, thermal behavior, detonation performance and application of FOX-7[2-4].
FOX-7 can react with some nucleophiles to synthesize new high energetic derivatives due to its "push-pull" nitro-enamine structure, which possesses a highly polarized carbon-carbon double bond with positive and negative charges stabilized by the amino group and nitro group respectively[5-7]. The positive end of the carbon-carbon double bond can be attacked by nucleophilic reagents to synthesize new single substituted derivatives or disubstituted closed-loop derivatives. We have synthesized many derivatives of FOX-7, such as 1-amino-1-hydrazino-2, 2-dinitroethene(AHDNE), 1-amino-1-(2, 4-dinitrophenylhydrazinyl)-2, 2-dinitroethylene(APHDNE), 1-amino-1-methylamino-2, 2-dinitroethylene(AMFOX-7), 2-(dinitromethylene)-1, 3-diazacyclopentane(DNDZ), 5-(dinitromethylene)-tetrazole (DNMT), series of potassium and other salts or complexes[8-13], some of which will be used as energetic catalysts or energetic flame suppressor in propellant.
1-Amino-1-ethylamine-2, 2-dinitroethene(AEFOX-7) was a derivative synthesized by our group in 2010, whose synthesis, theoretical calculation and thermal behavior have been reported [13]. But the crystal structure of AEFOX-7 was not got by many methods. However, we obtain it with an unexpected method. This paper mainly reports the crystal structure and enthalpy of combustion of AEFOX-7.
2 Experiments 2.1 SampleFOX-7 was obtained from Xi′an Modern Chemistry Research Institute. AEFOX-7 and AMFOX-7 were synthesized according to References [13-14]. The purities of the three compounds are all over 99%.
2.2 Determination of the single crystal structureWe tried to prepare Cd(ethamine)2(FOX-7)2 by putting Cd(NO3)2 and K(FOX-7)·H2O into ethylamine aqueous solution (50%). Although there was no success of Cd(ethamine)2(FOX-7)2, we finally got many crystals of AEFOX-7·H2O. In the system, substitution reaction of FOX-7 happened at the catalytic action of Cd2+ at room temperature for 3 months.
The crystal with dimensions of 0.39 nm×0.21 nm ×0.19 mm was chosen for X-ray determination. The data were collected on a Bruker SMART APEX CCD X-ray diffractometer using graphite-monochromated Mo Kα radiation (λ=0.071073 nm). The structure was solved by the direct methods (SHELXTL-97) and refined by the full-matrix-block least-squares method on F2 with anisotropic thermal parameters for all non-hydrogen atoms[15-16]. The hydrogen atoms were added according to the theoretical models. Crystal data and refinement results of AEFOX-7·H2O are summarized in Table 1. CCDC No.: 1042545.
| Tab.1 Crystal data and structures refinement details |
Enthalpy of combustion was determined by IKA C5000 oxygen-bomb calorimeter (German) adiabatically. The calorimeter was calibrated with the standard substance benzoic acid having a purity of 99.99%, and each sample was tested with 6 times. The calibration result of -(26503±73.30) J·g-1 for the standard substance benzoic acid is very close to the standard value as -(26434±5.8) J·g-1 at 298.15 K, indicating that the measuring system is accurate and reliable [17].
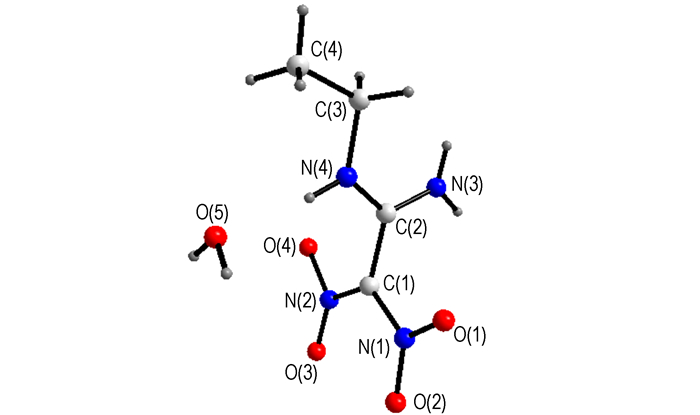
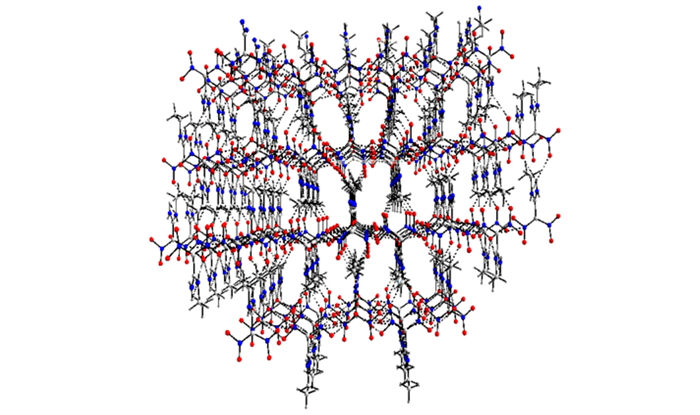
3 Results and Discussion 3.1 Crystal StructureMolecular structure and crystal packing of AEFOX-7·H2O are illustrated in Fig. 1 and Fig. 2. Selected bond lengths and bond angles of AEFOX-7·H2O are listed in Table 2.
|
Fig.1 Molecular structure of AEFOX-7·H2O |
|
Fig.2 Crystal packing of AEFOX-7·H2O |
| Tab.2 Selected bond lengths and bond angles of AEFOX-7·H2O |
The analytical results indicate that the molecule of AEFOX-7·H2O is made up of an amino group, an ethyl-amino group, two nitryls and a crystal water as shown in Fig. 1. There are three orthogonal planes in the molecule of AEFOX-7: the nitro-nitro groups plane, the amino-imino groups plane and the ethyl group plane, the intersection of the first two planes is C(1)—C(2), while N(4)—C(3) combines the last two planes, and the plane to which the crystal water belongs parallels with the nitro-nitro groups plane. Comparing the results with that of the parent compound FOX-7 and other derivatives, such as 1-amino-1-methylamino-2, 2-dinitroethylene (AMFOX-7), one amino group is substituted by the nucleophile of methylamine. Consequently, the molecular symmetry is broken in a certain extent, the molecular configuration changes and the molecular structure almost transforms into own two orthogonal planes (the nitro-nitro groups plane and the amino-methylamino groups plane).
In accordance with the structural analysis, there is a hydrogen bond in the molecule. Each molecule of AEFOX-7 forms four hydrogen bonds, which are formed by the hydrogen atoms of the crystal water and connecting two adjacent nitryls of two adjoining molecules, with neighboring molecules to form an infinite 1D chain. The adjacent two 1D chains are attached by two kinds of hydrogen bonds to form a 2D monolayer network. These two sorts of hydrogen bonds appear between the amino groups of one 1D chain and the nitryls of the neighboring chain. The crystal packing (Fig. 2) is formed by weak N—H…O interactions between adjacent 2D layers. The details of the above hydrogen bonds are shown in Table 3.
| Tab.3 Hydrogen bonds of AEFOX-7·H2O |
From Table 2, we can see that the bond length of C(1)—C(2) (0.14877 nm) is longer than the average length of C—C double bond (0.132 nm) and close to the length of C—C single bond (0.153 nm). The bond lengths of C(2)—N(3) and C(2)—N(4) (all equal to 0.130 nm approximately) are shorter than the average length of C—N single bond (0.147-0.150 nm), and close to the length of conjugated C—N double bond (0.128 nm). All these prove that the obvious conjugative effect occurs between bond C(1)—C(2), C(2)—N(3), and C(2)—N(4).
Comparing related bond lengths of EAFOX-7 with that of FOX-7, it can be seen that the change is great[5].The bond length of C(1)—C(2) (0.14877 nm) is longer than that of FOX-7 as 1.454 nm. Bond C(1)—N(1) and C(1)—N(2) change greatly from 0.1426, 0.1387 nm in FOX-7 to 0.1396, 0.1352 nm in EAFOX-7. Corresponding C(2)—N(3) and C(2)—N(4) bond shorten form 0.1317, 0.1313 nm to 0.13077, 1.3004 nm. The introduction of ethyl group (electron-donating group) has an obvious effect on the configuration of molecule. The whole molecule presents a shift trend from amino groups to nitro groups. The introduction of ethyl group makes the "push-pull" effect enhance, also making EAFOX-7 exhibit many different physicochemical properties with FOX-7[13].
3.2 Constant-volume Combustion EnthalpyFrom the determination results in Table 4, it can be seen that theconstant-volume combustion enthalpies for AEFOX-7, AMFOX-7 and FOX-7 are -(13327.83±27.46), -(11225.33±36.65) J·g-1 and -(7831.00±8.75) J·g-1, respectively. The sequence of enthalpy of combustion for these compounds is AEFOX-7>AMFOX-7>FOX-7. So, the enthalpy of combustion rises with the increase of the number of C atom in molecule.
| Tab.4 Determination results of enthalpy of combustion |
The standard molar enthalpy of combustion (ΔcHmθ) was referred to the combustion enthalpy change of the following idealized reaction formula (1) at T=298.15 K and pθ=101.325 kPa[18].
| $ {\rm{M(s) + }}\mathit{a}{{\rm{O}}_2}({\rm{g}}) = \mathit{b}{\rm{C}}{{\rm{O}}_2}({\rm{g}}) + \mathit{c}{{\rm{H}}_2}{\rm{O(l) + }}\mathit{d}{{\rm{N}}_2}({\rm{g}}) $ | (1) |
M=AEFOX-7: C4H8O4N4, a=4, b=4, c=4, d=2;
AMFOX-7: C3H6O4N4, a=5/2, b=3, c=3, d=2;
FOX-7: C2H4O4N4, a=1, b=2, c=2, d=2.
ΔcHmθ can be derived from the standard molar enthalpy of combustion in accordance with equations (2) and (3). The calculated results are listed in Table 5.
| $ {\Delta _{\rm{c}}}H_{\rm{m}}^{\rm{ \mathsf{ θ} }} = {\Delta _{\rm{c}}}U_{\rm{m}}^{\rm{ \mathsf{ θ} }} + \Delta nRT $ | (2) |
| $ \Delta n = \sum {{n_{\rm{i}}}({\rm{products,g}})} - \sum {{n_{\rm{i}}}({\rm{reactants}},{\rm{g}})} $ | (3) |
| Tab.5 Standard molar enthalpies of constant-volume combustion, standard molar enthalpies of combustion and standard molar enthalpies of formation of the three compounds at 298.15 K |
where ∑ni is the total molar amount of gases in products or reactants.
The standard molar enthalpy of formation of the compound(ΔfHmθ) can be calculated by Hess′s law [18], according to the above thermochemical equation. Taking AEFOX-7 for instance, the standard molar enthalpy of formation is:
ΔfHmθ(AEFOX-7, s)= 4ΔfHmθ(CO2, g)+4ΔfHmθ(H2O, l)-ΔcHmθ(AEFOX-7, s)
The standard molar enthalpies of formation for CO2(g) and H2O (l) recommended by CODATA, ΔfHmθ (CO2, g)=-(393.51±0.13) kJ·mol-1 and ΔfHmθ (H2O, l)=-(285.83±0.04) kJ·mol-1, are employed in the calculation of ΔfHmθ(compound, s) values[17-18]. The standard molar enthalpies of formation for AEFOX-7, AMFOX-7 and FOX-7 can be calculated and listed in Table 5, and the results also indicate that the standard molar enthalpy of formation rises with the number of C atom in molecule. Moreover, the standard molar enthalpy of formation of FOX-7 [-(206.35±1.33) kJ·mol-1] is close to the literature value obtained by theoretical calculation -133.7 kJ·mol-1[19].
4 ConclusionsThe single crystal of AEFOX-7·H2O is obtained unexpectedly. The crystal is orthorhombic with space group of Pna2(1)/m. The enthalpies of combustion of AEFOX-7, AMFOX-7 and FOX-7 are measured to be -(2347.83±4.84), -(1819.96±5.94), -(1159.77±1.30) kJ·mol-1 respectively. Their corresponding standard molar enthalpies of formation are -(374.49±4.87), -(224.25±5.95) and -(206.35±1.33) kJ·mol-1, respectively. Both enthalpies of combustion and standard molar enthalpies of formation rise with the increase of the number of C atom in molecule.
| [1] |
Latypov N V, Bergman J, Langlet A, et al. Synthesis and reaction of 1, 1-diamino-2, 2-dinitroethylene[J]. Tetrahedron, 1998, 54: 11525-11536. DOI:10.1016/S0040-4020(98)00673-5 |
| [2] |
Ek S, Ottis J, Dudek K, Ruzicka A, et al. Scalable synthesis of 1, 1-diamino-2, 2-dinitroethene without hazardous intermediates or by-products[J]. Journal of Energetic Materials, 2013, 54: 87-99. |
| [3] |
Dreger Z A, Zbigniew A, Tao Y C, et al. High-pressure vibrational and polymorphic response of 1, 1-diamino-2, 2-dinitroethene single crystals: raman spectroscopy[J]. Journal of Phyical Chemistry A, 2014, 118: 5002-5012. DOI:10.1021/jp5052062 |
| [4] |
Zabka J, Simkova L, Jalovy Z, et al. The unimolecular chemistry of protonated and deprotonated 2, 2-dinitroethene-1, 1-diamine (FOX-7) studied by tandem mass spectrometry and computational chemistry[J]. European Journal of Mass Spectrometry, 2014, 20: 233-247. DOI:10.1255/ejms.1273 |
| [5] |
Bemm U, Östmark H. 1, 1-Diamino-2, 2-dinitroethylene: a novel energetic material with infinite layers in two dimensions[J]. Acta Crystallographica Section C, 1998, 54: 1997-1999. |
| [6] |
Herve G, Guy J, Latypov N. The reactivity of 1, 1-diamino-2, 2-dinitroethene (FOX-7)[J]. Tetrahedron, 2005, 61: 6743-6748. DOI:10.1016/j.tet.2005.05.010 |
| [7] |
Anniyappan M, Talawar M B, Gore G M, et al. Synthesis, characterization and thermolysis of 1, 1-diamino-2, 2-dinitroethylene(FOX-7) and its salts[J]. Journal of Hazardous Materials, 2006, 137: 812-819. DOI:10.1016/j.jhazmat.2006.03.034 |
| [8] |
Xu K Z, Song J R, Yang X, et al. Molecular structure, theoretical calculation and thermal behavior of 2-(1, 1-dinitromethylene)-1, 3-diazepentane[J]. Journal of Moecular Structure, 2008, 891: 340-345. DOI:10.1016/j.molstruc.2008.04.004 |
| [9] |
Chang C R, Xu K Z, Song J R, et al. Preparation, crystal structure and theoretical calculation of 1-amino-1-hydrazino-2, 2-dinitroethene[J]. Acta Chimica Sinica, 2008, 66: 1399-1404. |
| [10] |
Xu K Z, Chang C R, Song J R, et al. Preparation, crystal structure and theoretical calculation of G(FOX-7)[J]. Chinese Journal of Chemistry, 2008, 26: 495-499. DOI:10.1002/(ISSN)1614-7065 |
| [11] |
Xu K Z, Song J R, Zhao F Q, et al. Non-isothermal decomposition kinetics, specific heat capacity and adiabatic time-to-explosion of a novel high energy material: 1-amino-1-methylamino-2, 2-dinitroethylene (AMFOX-7)[J]. Journal of the Chinese Chemical Society, 2009, 56: 524-531. DOI:10.1002/jccs.v56.3 |
| [12] |
Xu K Z, Zhao F Q, Song J R, et al. Non-isothermal decomposition kinetics of a new high-energy organic potassium salt: K(DNDZ)[J]. Bulletin of the Korean Chemical Society, 2009, 30: 2259-2264. DOI:10.5012/bkcs.2009.30.10.2259 |
| [13] |
Xu K Z, Zhao F Q, Wang F, et al. Structural characterization and thermal properties of 1-amino-1-ethylamino-2, 2-dinitroethylene[J]. Chinese Journal of Chemical Physics, 2010, 23: 335-341. DOI:10.1088/1674-0068/23/03/335-341 |
| [14] |
Xu K Z, Wang F, Ren Y H, et al. Structural characterization and thermal behavior of 1-amino-1-methylamino-2, 2-dinitroethylene[J]. Chinese Journal of Chemistry, 2010, 28: 583-588. DOI:10.1002/cjoc.201090116 |
| [15] |
Sheldrick G M. SHELXS[CP]. University of Göttingen, Germany, 1997.
|
| [16] |
Sheldrick G M. SHELXL[CP]. University of Göttingen, Germany, 1997.
|
| [17] |
Cox J D. CODATA recommended key values for thermodynamics, 1977 Report of the CODATA Task Group on key values for thermodynamics[J]. Journal of Chemical Thermodynamics, 1978, 10: 903-906. DOI:10.1016/0021-9614(78)90050-2 |
| [18] |
Atkins P, Paula J D. Atkins' Physical Chemistry (7th)[M]. Beijing: High Education Press, 2006.
|
| [19] |
Tian Y D, Zhao F Q, Liu J H. Handbook of Energetic Materials and the Related Compounds[M]. Beijing: National Defense Industry Press, 2011.
|
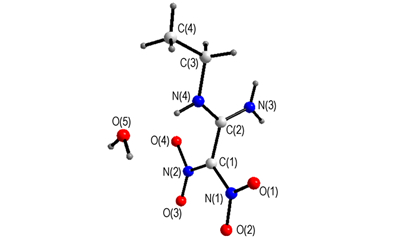
The single crystal of 1-amino-1-ethylamino-2, 2-dinitroethylene monohydrate (AEFOX-7·H2O) was obtained. The enthalpies of combustion of AEFOX-7, AMFOX-7(1-amino-1-methylamino-2, 2-dinitroethylene) and FOX-7 at 298.15 K were determined.




