In the field ofhigh energy density materials (HEDMs), the synthesis and development of new energetic materials continue to focus on new heterocycles with high density, high heat of formation, and good oxygen balance (index of the deficiency or excess of oxygen in a compound required to convert all carbon into carbondioxide, and all hydrogen into water) [1]. Of particular interest is high-nitrogen structure unit (e.g. triazole) in combination with energetic substituents such as nitro (—NO2), nitrato (—ONO2), azido (—N3) and nitramino (—NHNO2) functionalities, because these compounds have satisfactory detonation performance [2-6].
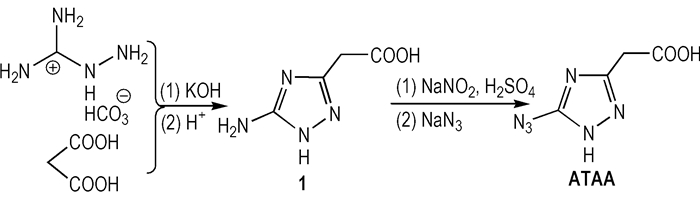
In thisarticle, a novel azido-triazole compound, 5-azido-1, 2, 4-triazolyl-5-acetic acid (ATAA), was synthesized by Sandmeyer-Reaction from 5-amino-1, 2, 4-triazolyl-5-acetic acid (1) for the first time (Scheme 1). Its structure characterization was performed using infrared (IR), mass spectrum (MS) and elemental analysis as well as multinuclear NMR spectroscopy. The thermal behaviors of ATAA were studied by thermal gravity analysis (TG) and differential scanning calorimetry (DSC). In addition, 5-azido-3-trinitromethyl-1 H-1, 2, 4-triazole (2), a novel polynitro-triazole energetic compound derived from ATAA, was designed and calculated. The energetic properties of 2 compared to HMX and its analogue 5-nitro-3-trinitromethyl-1 H-1, 2, 4-triazole (2i) of literature [6].
|
Scheme1 |
All chemical reagents and solvents were obtained from Sigma-Aldrich Inc. or ChengduKelong Reagent Company (analytical grade) and used as supplied without further purification. 5-amino-1, 2, 4-triazolyl-5-acetic acid (1) was prepared according to the literature [6] procedure. Infrared spectra were obtained using a Nexus 87 Fourier transform infrared spectroscope (Nicolet USA). Organic elemental composition was analyzed on Vario EL Ⅲ elemental analyzer (Elementar Germany). Purity analysis with HPLC was carried out on a LC-20A system equipped with a C18 column (250 mm×4.6 mm, 5 mm, Agela) and a UV detector set at 254 nm (Shimadzu Japan). 1H NMR (500.13 MHz) and 13C NMR (125.76 MHz) spectra were recorded on a V500 spectrometer (Bruker, Germany). Chemical shifts were reported as a δ value in parts per million and tetramethylsilane was used as the internal standard. 1H NMR and 13C NMR spectra were recorded in DMSO-d6.
2.2 Synthesis of ATAAA solution ofsodium nitrite (0.72 g, 10.5 mol, 2.1 equiv) in water (10 mL) was added dropwise to a suspension of 1 (0.71 g, 5 mmol) in 20% sulfuric acid (40 mL) at -10 ℃. The mixture was allowed to warm to -5 ℃ and subsequently a solution of sodium azide (0.5 g, 7.5 mmol, 1.5 equiv) in water (5 mL) was added dropwise for 10 min. Then the suspension was stirred for 2 h at -5 ℃. The pink solid was filtered and recrystallized in ethyl acetate. Collection of the colorless precipitate afforded ATAA·H2O, yielding 0.48 g. The purity was 97.5%. 1H NMR (DMSO-d6): δ 3.78 (s, 2H), δ 12.39 (s, 1H), 13.81 (s, 1H). 13C NMR (DMSO-d6): δ 169.3, 156.2, 151.6, 32.6. IR (KBr, ν/cm-1):3304 (vs), 2959 (w), 2445 (w), 2148 (vs), 1669 (s), 1550 (m), 1518 (vs), 1442 (m), 1389 (m), 1342 (w), 1263 (w), 1236 (vs), 1138 (w), 1059 (m), 1025 (w), 948 (w), 926 (w), 791 (m), 735 (m), 670 (w), 531 (w), 467 (w), 406 (w). MS (DEI+): m/z: 167.0 [C4H3N6O2]-. Elemental analysis (%) calcd for C4H4N6O2·H2O: C 25.81, H 3.25, N 45.15. Found: C 25.84, H 3.24, N 45.13.
2.3 Thermal Decomposition ConditionTGA-DSC was conducted on a TGA/DSC 2 STARe system (Mettler, Switzerland) under an argon atmosphere at a flow rate of 100 mL·min-1. The sample mass was 0.7 mg and the heating rate was 10 ℃·min-1. The temperature range was from room temperature to 300 ℃.
3 Results and Discussions 3.1 SynthesesThe synthesis starts with commercially available1, which was synthesized by intramolecular condensation of malonic acid and aminoguanidine bicarbonate in alkaline medium[7]. In general the azidation reaction is performed at 0-5 ℃[8-9]. According to the previously published azidation process, ATAA was synthesized tentatively from 1 through diazotization in sodium nitrite and sulfuric acid at 0 ℃ and subsequent reaction with an excess of sodium azide at 5 ℃. However, a mass of bright red precipitate was generated among the reaction progress. The precipitate was analyzed by thin layer liquid chromatography (TLC), and included three components. These results suggest that the diazonium derived from 1 is instable and highly reactive at the above temperature range, causing the decrease of reaction selectivity. We infer that decreasing the reaction temperature may have a protective effect on the azidation reaction through reducing side reaction (e.g. azo reaction). Therefore, the diazotization process and the azidation process were controlled at lower temperatures of -10 ℃ and -5 ℃. The bright red precipitate was significantly reduced as expected. ATAA was obtained as colorless precipitate, which can be purified by recrystallization in ethyl acetate. The complete synthetic process was shown in Scheme 1.
3.2 Thermal Behavior of ATAAAs shown in the TGA curve (Fig. 1), it indicates the thermal behavior of ATAA can be divided into one dehydrating crystal water stage and one obvious decomposition stage. The dehydration occurs at 63.9-109.3 ℃ with a mass loss of about 9.7%, corresponding to the loss of 1 mol water molecule. And this result, which is consistent with that of elemental analysis, has proven further that the exact chemical formula of the title compound is ATAA·H2O. The exothermic decomposition starts at 158.3 ℃ and ends at 201.2 ℃ with a mass loss of about 66.6%. The TGA curve shows almost 80% mass loss.

|
Fig.1 TG curves of ATAA at a heating rate of 10.0 ℃·min-1 |
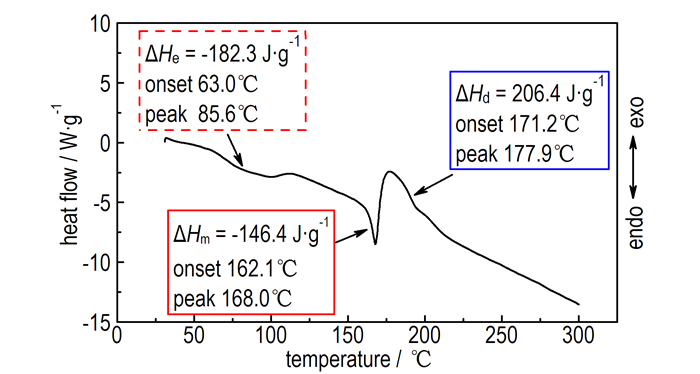
From Fig. 2, we can see the DSC curve of ATAA is in harmony with the TGA curve, including two endothermic processes and one exothermic process. The first endothermic process is corresponding to the dehydrating crystal water stage, and the dehydration enthalpy (ΔHe), the extrapolated onset temperature as well as the peak temperature are -182.3 J·g-1, 63.0 ℃ and 85.6 ℃, respectively. The second endothermic process belongs to the melting stage. The melting enthalpy (ΔHm), the extrapolated onset temperature and the peak temperature are -146.4 J·g-1, 162.1 ℃ and 168.0℃. The exothermic process is derived from the decomposition of ATAA, and the decomposition enthalpy (ΔHd), the extrapolated onset temperature and the peak temperature are 206.4 J·g-1, 171.2 ℃ and 177.9 ℃. The foregoing data of TGA and DSC shows that the thermal stability of ATAA is excellent among the azido-1, 2, 4-triazole derivates [10-11], which may be due to the strong hydrogen bond system between N—H of triazole and —COOH.
|
Fig.2 DSC curve of ATAA at a heating rate of 10.0 ℃·min-1 |
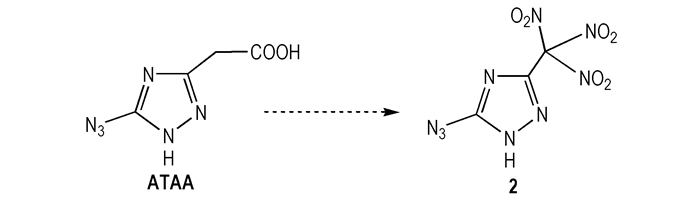
Highly energetic compounds which have polynitro groups are one of the important classes of useful energetic materials[6, 12]. And azole acetic can be converted into corresponding trinitromethyl azoles by nitration with mixed acids, HNO3 and H2SO4[13]. As such, 5-azido-3-(trinitromethyl)-1H-1, 2, 4-triazole (2), the molecule displayed in Scheme 2, is potential via nitrification reaction step from ATAA. The detonation performances of 2 were calculated by Monte-Carlo method[14], Atomization scheme [15] and Kamlet-Jacobs formula[16]. These data was listed in the Table 1. The calculated densities, the detonation pressure, as well as the detonation velocity is 1.91 g·cm-3, 38.67 GPa and 9096 m·s-1, respectively. These calculated data indicates the detonation performance of 2 is comparable to HMX and its analogues 2i [6]. Additionally, 2 has obvious advantage in the stand enthalpies of formation and nitrogen content compare to HMX and 2i. Our efforts toward the synthesis of the excellent material are currently on going.
|
Scheme2 |
| Tab.1 Prediction of performance for 2 and 3 |
A novel azido-triazole, ATAA, was synthesized for the first time by Sandmeyer-Reaction from 1. Typical TG and DSC curve indicates the thermal behavior of ATAA includes one dehydrating crystal water stage, one melting stage and one obvious melting decomposition stage. And the peak temperatures at each stage are 85.6 ℃, 168.0 ℃ and 177.9 ℃. The detonation performance of 2 derived from ATAA was calculated. The stand enthalpies of formation, the calculated densities as well as the detonation velocity belonging to 2 are 449.62 kJ·mol-1, 1.91 g·cm-3 and 9096 m·s-1, respectively.
| [1] |
Ye C, Gao H, Boatz J A, et al. Polyazidopyrimidines: high-energy compounds and precursors to carbon nanotubes[J]. Angewandte Chemie International Edition, 2006, 45(43): 7262-7265. DOI:10.1002/(ISSN)1521-3773 |
| [2] |
Dippold A A, Klapötke T M. A Study of dinitro-bis-1, 2, 4-triazole-1, 1'-diol and derivatives-design of high performance insensitive energetic materials by the introduction of N-oxides[J]. Journal of the American Chemical Society, 2013, 135(26): 9931-9938. DOI:10.1021/ja404164j |
| [3] |
Dippold A A, Izsák D, Klapötke T M. A study of 5-(1, 2, 4-triazol-C-yl) tetrazol-1-ols: combining the benefits of different heterocycles for the design of energetic materials[J]. Chemistry-A European Journa, 2013, 19(36): 12042-12051. DOI:10.1002/chem.201301339 |
| [4] |
Dippold A A, Klapötke T M, Winter N. Insensitive nitrogen-rich energetic compounds based on the 5, 5'-dinitro-3, 3'-bi-1, 2, 4-triazol-2-ide anion[J]. European Journal of Inorganic Chemistry, 2012, 18(21): 3474-3484. |
| [5] |
Thottempudi V, Shreeve J N M. Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1H-1, 2, 4-triazole and 5, 5'-bis(trinitromethyl)-3, 3'-azo-1H-1, 2, 4-triazole[J]. Journal of the American Chemical Society, 2011, 133(49): 19982-19992. DOI:10.1021/ja208990z |
| [6] |
Thottempudi V, Gao H, Shreeve J N M. Trinitromethyl-substituted 5-nitro-or3-azo-1, 2, 4-triazoles: synthesis, characterization, and energetic properties[J]. Journal of the American Chemical Society, 2011, 133(16): 6464-6471. DOI:10.1021/ja2013455 |
| [7] |
A bdel-Megeed A M, Abdel-Rahman H M, Alkaramany G E, et al. Design, synthesis and molecular modeling study of acylated 1, 2, 4-triazole-3-acetates with potential anti-inflammatory activity[J]. European Journal of Medicinal Chemistry, 2009, 44(1): 117-123. DOI:10.1016/j.ejmech.2008.03.017 |
| [8] |
Izsák D, Klapötke T M. Preparation and crystal structure of 5-azido-3-nitro-1H-1, 2, 4-triazole, its methyl derivative and potassium salt[J]. Crystals, 2012, 2(2): 294-305. |
| [9] |
Dippold A A, Klapötke T M. Nitrogen-rich bis-1, 2, 4-triazoles-a comparative study of structural and energetic properties[J]. European Journal of Medicinal Chemistry, 2012, 18(52): 16742-16753. DOI:10.1002/chem.v18.52 |
| [10] |
Izsák, Dániel, Klapötke, Thomas M Scharf, Regina. Energetic Materials Based on the 5-Azido-3-nitro-1, 2, 4-triazolate Anion[J]. Zeitschrift Für Anorganische Und Allgemeine Chemie, 2013, 10(639): 1746-1755. |
| [11] |
LI Ya-yu, PANG Si-ping, SUN Cheng-hui. Synthesis and crystal structure of a nitrogen-rich compound: 3, 5-diazido-1, 2, 4-triazole[J]. Journal of Beijing Institute of Technology, 21(3): 421-426.
|
| [12] |
Kettner M, Klapötke T M. 5, 5'-Bis-(trinitromethyl)-3, 3'-bi-(1, 2, 4-oxadiazole): a stable ternary CNO-compound with high density[J]. Chemical Communications, 2014, 50(18): 2268-2270. DOI:10.1039/C3CC49879D |
| [13] |
Thottempudi V, Kim T K, Chung K H, et al. Synthesis and characterization of some polynitro imidazoles[J]. ChemInform, 2010, 41(7): 2152-2154. |
| [14] |
BI Fu-qiang, FAN Xue-zhong, XU Cheng, et al. Synthesis and theoretical study of 1, 1'-dihydroxy-5, 5'-bitetrazole[J]. Chinese Journal of Explosives & Propellants, 2013, 36(4): 22-25. |
| [15] |
Curtiss L A, Raghavchari K, Redfern P C, et al. Gaussian-3 (G3) theory for molecules containing first and second-row atoms[J]. The Journal of Chemical Physics, 1998, 109(18): 7764-7776. DOI:10.1063/1.477422 |
| [16] |
Kamlet M J, Jacobs S. Chemistry of detonations. I. A simple method for calculating detonation properties of C—H—N—O explosives[J]. The Journal of Chemical Physics, 1968, 48(1): 23-35. DOI:10.1063/1.1667908 |
| [17] |
Meyer R, Homburg A. Explosives[M]. Wiley, 2007, 156-157.
|
A novel azido-triazole, 5-azido-1, 2, 4-triazolyl-5-acetic acid(ATAA), was synthesized for the first time by Sandmeyer-Reaction in a mixed system (including sodium nitrite, sulfuric acid and sodium azide).




