2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China
2. 西安近代化学研究所, 陕西 西安 710065
1, 1-Diamino-2, 2-dinitroethylene (FOX-7) is a high-energy material with high thermal stability and low sensitivity to impact and friction[1-2]. Since first reported in 1998, FOX-7 has been considered as a research emphasis of energetic materials and will be used in insensitive ammunition and solid propellant. FOX-7 is a representative "push-pull" nitro-enamine, which possesses a highly polarized carbon-carbon double bond with positive and negative charges being stabilized by the amino group and nitro group respectively, and presents certain acidic properties[3-9]. Many researches have been studied on the synthesis[5-6], mechanism[4], molecule structure[2], theoretical calculation[11], thermal behavior[12], explosive performance and application[28] of FOX-7. Existing in manifold tautomers and resonances, FOX-7 can react with some nucleophiles to prepare many new energetic derivatives[11]. Some energetic salts, such as potassium salt, rubidium salt, cesium salt and guanidine salt, have been reported[11-12]. Other salts and metal complexes of FOX-7 also can be synthesized through replacement reaction, such as Cu(NH3)2(FOX-7)2, Cu(CH3NH3)2(FOX-7)2, [Cu(en)2(FOX-7)2(H2O)]·H2O, [Cu(phen)2FOX-7] Cl·3H2O, Zn(NH3)2(FOX-7)2 and Zn(en)2(FOX-7)2[14-17].
Many energetic Cu(Ⅱ) complexes were often used as detonating explosive or combustion catalyst of solid propellant[18-21], so we hope that Cu-FOX-7 complexes can also be used as energetic catalyst. Cu(pn)2(FOX-7)2 is a new typical FOX-7 complex, and its synthesis and crystal structure have been reported[16]. In this paper, we studied the decomposition kinetics of Cu(pn)2(FOX-7)2, determined specific heat capacity and calculated adiabatic time-to-explosion for further estimating its thermal stability.
2 Experimental 2.1 SynthesisAll chemicals used in synthesis were analytical-grade commercial products. FOX-7 came from Xi'an Modern Chemistry Research Institute (purity>99%). K(FOX-7)·H2O was prepared according to the ref.[13].
Cu(pn)2(FOX-7)2 (pn=1, 3-diaminopropane) was prepared according to ref.[16] as follows: K(FOX-7)·H2O (2 mmol) and Cu(NO3)2·3H2O (1 mmol in 3 mL water) were stirred in 1, 3-diaminopropane solution (78 mmol) for 30 min to give a clear solution at room temperature. Gradually purple crystals slowly appeared and were identified as Cu(pn)2(FOX-7)2. (yield 46%, 0.233 g). IR (KBr, ν/cm-1): 3408, 3294, 3226, 2931, 2359, 2025, 1659, 1500, 1344, 1281, 1132, 1029, 926, 829, 741, 681, 501. Anal. Calcd.(%) for C10H26N12O8Cu: C 23.21, H 5.32, N 33.68; found: C 23.14, H 5.57, N 33.22.
2.2 Physical MeasurementsThe DSC experiments were performed using a DSC200 F3 apparatus (NETZSCH, Germany) under a nitrogen atmosphere at a flow rate of 80 mL·min-1. The heating rates were 5.0, 7.5, 10.0 ℃·min-1 and 12.5 ℃·min-1 from ambient temperature to 350 ℃, respectively. The TG/DTG experiment was performed using a SDT-Q600 apparatus (TA, USA) under a nitrogen atmosphere at a flow rate of 100 mL·min-1. The heating rate was 5.0 ℃·min-1 from ambient temperature to 350 ℃. The specific heat capacity was determined using a Micro-DSCⅢ apparatus (SETARAM, France). The heating rate used was 0.15 ℃·min-1 from 10 ℃ to 80 ℃. The sample mass was 115.7 mg.
The impact sensitivity was determined by using a ZBL-B impact sensitivity instrument (NACHEN, China). The mass of drop hammer is 2.5 kg. The sample mass for every test is 30 mg.
3 Results and Discussion 3.1 Thermal Decomposition BehaviorDSC curves of Cu(pn)2(FOX-7)2 at various heating rates are shown in Fig. 1. TG-DTG curve of Cu(pn)2(FOX-7)2 sample at a heating rate of 5.0 ℃·min-1 is given in Fig. 2.

|
Fig.1 DSC curves of Cu(pn)2(FOX-7)2 |

|
Fig.2 TG/DTG curve of Cu(pn)2(FOX-7)2 at 5 ℃·min-1 |
Fig. 1 shows that the DSC curves of Cu(pn)2(FOX-7)2 exhibit two exothermic peaks, which are in agreement with the results of TG/DTG, and the peak temperatures go up with the increase of heating rate. Fig. 2 illustrates that the thermal decomposition of Cu(pn)2(FOX-7)2 can be divided into two decomposition processes. The first is an intense decomposition process, which occurs at 140-185 ℃ with a mass loss of 35.30%. The extrapolated onset temperature, peak temperature and heat of decomposition are 155.47 ℃, 156.49 ℃ and 816.5 J·g-1 at the heating rate of 5.0 ℃·min-1. The second stage is a slow decomposition process at the temperature range of 185-270 ℃ with a mass loss of about 14.19%. The peak temperature is 215.8 ℃ at a heating rate of 5.0 ℃·min-1. The final residue at 350 ℃ is about 41.74%. Comparing with the thermal decomposition of Cu(NH3)2(FOX-7)2[14], they exhibits similar thermal decomposition processes, but the thermal stability of Cu(pn)2(FOX-7)2 is slightly lower than that of Cu(NH3)2(FOX-7)2, which is due to the introduce of long carbon chain.
3.2 Non-isothermal Decomposition KineticsIn order to obtain the kinetic parameters(the apparent activation energy (E) and pre-exponential factor (A)) of the first exothermic decomposition process, Kissinger method[21] and Ozawa method[22] were employed. The determined values of the beginning temperature (T0), extrapolated onset temperature (Te) and peak temperature (Tp) at the different heating rates are listed in Table 1. The values of T00 and Te0[22]corresponding to β→0 obtained by Eq. (1) are also listed in Table 1.
| $ {T_{{\rm{0}}\mathit{i}\;{\rm{or}}\;{\rm{e}}\mathit{i}}} = {T_{00\;{\rm{or}}\;{\rm{e0}}}} + n{\beta _i} + m{\beta _i}, \;\;\;\;\;i = 1-4 $ | (1) |
| Tab.1 The values of T0, Te, Tp, T00, Te0 and kinetic parameters of the first exothermic decomposition process for Cu(pn)2(FOX-7)2 determined from DSC curves at various heating rates (β) |
where n and m are coefficients.
The calculated kinetic parameters (E and A) in Table 1 show that the E obtained by Kissinger method is consistent with that by Ozawa method. The linear correlation coefficients (r) are all close to 1. So, the result is credible.
T versus α (the conversion degree) curves at different heating rates are shown in Fig. 3. The values of EO for any given value of α were obtained and shown in Fig. 4. The values of EO steadily distribute from 142 to 158 kJ·mol-1 in the α range of 0.175-0.875, and the average value of EO is 151.9 kJ·mol-1, which is in approximate agreement with that obtained by Kissinger method and Ozawa method from only peak temperature values (163.5 and 162.3 kJ·mol-1, respectively). So, the values were used to check the validity of E by other methods.
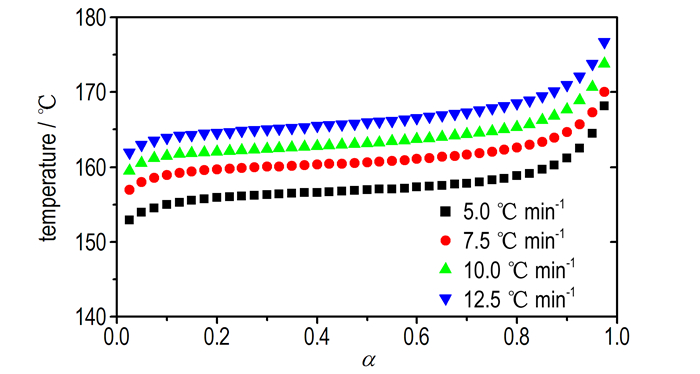
|
Fig.3 T vs α curves for the decomposition reaction of Cu(pn)2(FOX-7)2 at different heating rates |
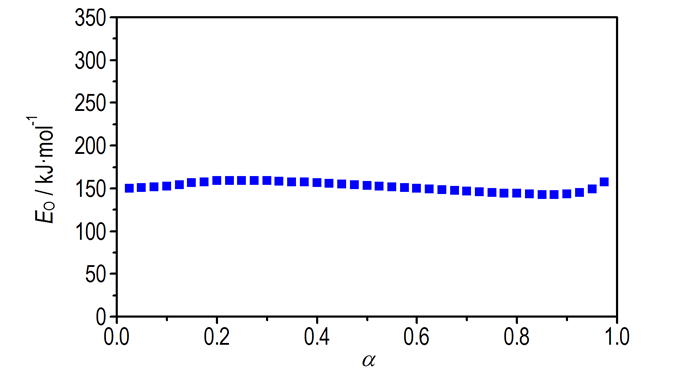
|
Fig.4 EO vs α curve for the decomposition reaction of Cu(pn)2(FOX-7)2 by Ozawa method |
The integral equations (The general integral equation, The universal integral equation, MacCallum-Tanner equation, Šatava-Šesták equation and Agrawal equation) were cited to obtain the values of E, A and the most probable kinetic model function [f(α)] from each DSC curve[24]. Forty-one types of kinetic model functions taken from Ref. [24] and experimental data form each DSC curve were put into the above five integral equations for calculating, respectively. The values were obtained and shown in Table 2. So, the most probable kinetic model function is classified asf(α)=3α2/3(No. 23 equation, Mampel power law, n=1/3), according to the unanimity rule of calculation results from each model equation[24]. The kinetic equation can be described as:
| $ \frac{{{\rm{d}}\alpha }}{{{\rm{d}}\mathit{T}}} = \frac{{{{10}^{17.83}}}}{\beta }3{\alpha ^{2/3}}\exp (-1.635 \times {10^5}/\mathit{RT}) $ | (2) |
| Tab.2 |
The self-accelerating decomposition temperature (TSADT) and critical temperature of thermal explosion (Tb) are two important parameters required to ensure safe storage and process operations for energetic materials and then to evaluate the thermal stability[24-25]. TSADT and Tb can be obtained by Eq. (3) and Eq. (4), respectively.
| $ {\mathit{T}_{{\rm{SADT}}}} = {T_{{\rm{e0}}}} $ | (3) |
| $ {T_{\rm{b}}} = \frac{{{E_{\rm{O}}}-\sqrt {E_{\rm{O}}^2-4{E_{\rm{O}}}R{T_{{\rm{e0}}}}} }}{{2R}} $ | (4) |
TSADT and Tb for Cu(pn)2(FOX-7)2 are 145.6 ℃ and 146.7 ℃, respectively, which are similar with those of Cu(NH3)2(FOX-7)2 as 145.5 ℃ and 156.2 ℃[26], but much lower than those of FOX-7 as 206.0 ℃ and 207.1 ℃[27]. Admittedly, the thermal stability of FOX-7 all declines when it becomes salts or complexes, and the decomposition process also becomes severe.
3.4 Specific Heat CapacityFigure 5 shows the result of Cu(pn)2(FOX-7)2 measured by a continuous specific heat capacity mode of Micro-DSCⅢ. In determined temperature range, specific heat capacity presents a good quadratic relationship with temperature. Specific heat capacity equation of Cu(pn)2(FOX-7)2 is :
| $ \begin{array}{l} {c_p} =-2.6824 + 1.9441 \times {10^{-2}}T-2.0494 \times {10^{ - 5}}{T^2}\\ (285.0\;{\rm{K < }}\mathit{T < }{\rm{350}}{\rm{.0}}\;{\rm{K}}) \end{array} $ | (5) |
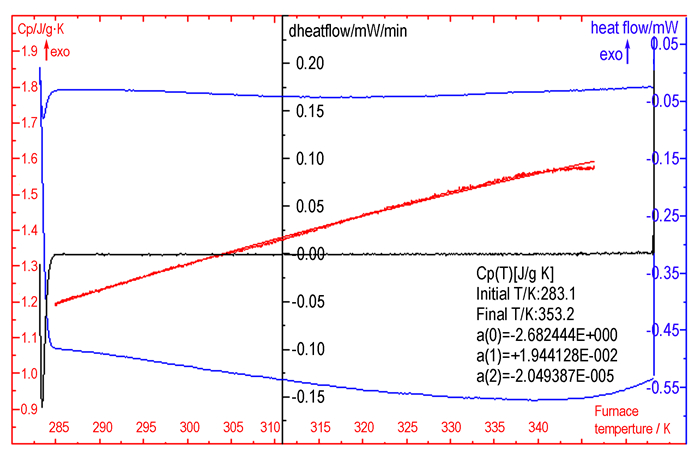
|
Fig.5 Determination results of the continuous specific heat capacity of Cu(pn)2(FOX-7)2 |
where cp is the specific heat capacity in J·g-1·K-1.
The molar heat capacity of Cu(pn)2(FOX-7)2 is 653.79 J·mol-1·K-1 at 298.15 K.
3.5 Adiabatic Time-to-explosionThe adiabatic time-to-explosion[24, 28] is also an important parameter for evaluating the thermal stability of energetic materials and can be calculated by Eqs. (6) and (7).
| $ {c_p}\frac{{{\rm{d}}\mathit{T}}}{{{\rm{d}}\mathit{t}}} = QA\exp (-\mathit{E}{\rm{/}}\mathit{RT})\mathit{f}{\rm{(}}\alpha {\rm{)}} $ | (6) |
| $ \alpha {\rm{ = }}\int_{{T_0}}^T {\frac{{{c_p}}}{Q}{\rm{d}}\mathit{T}} $ | (7) |
where T is the absolute temperature in K, t is the adiabatic time-to-explosion in s, Q is the exothermic values in J·mol-1, A is the pre-exponential factor in s-1, E is the apparent activation energy of the thermal decomposition reaction in J·mol-1, R is the gas constant in J·mol-1·K-1, f(α) is the most probable kinetic model function and α is the conversion degree.
The adiabatic time-to-explosion equation is:
| $ t = \frac{1}{{QA}}\int_{{T_0}}^T {\frac{{{c_{\rm{p}}}\exp (\mathit{E}{\rm{/}}\mathit{RT})}}{{f(\alpha )}}} {\rm{d}}\mathit{T} $ | (8) |
where the limit of temperature integration is from T00 to Tb.
In fact, the value of α of energetic materials from the beginning thermal decomposition to thermal explosion in the adiabatic conditions is very small, and it is very difficult to obtain the most probable kinetic model function [f(α)] at the process. So, Power-low model [Eq.(9)], Reaction-order model [Eq.(10)] and Avrami-Erofeev model [Eq.(11)] were separately used to estimate the adiabatic time-to-explosion [24, 29]. The calculation results are listed in Table 3.
| $ \mathit{f}{\rm{(}}\alpha {\rm{)}} = n{\alpha ^{(\mathit{n}{\rm{-1}})/\mathit{n}}} $ | (9) |
| $ \mathit{f}{\rm{(}}\alpha {\rm{)}} = {(1-\alpha )^\mathit{n}} $ | (10) |
| $ \mathit{f}{\rm{(}}\alpha {\rm{)}} = n(1- \alpha ){[-{\rm{ln}}(1-\alpha )]^{(\mathit{n}{\rm{ -1}})/\mathit{n}}} $ | (11) |
| Tab.3 The calculation results of adiabatic time-to-explosion |
From Table 3, we can see that the calculation results exhibit some deviation and the decomposition model has big influence on the estimating result of adiabatic time-to-explosion. Form the results, the adiabatic time-to-explosion of Cu(pn)2(FOX-7)2 is calculated to about 77 s. The time can be proved credible according to the change of DSC curves in the exothermic decomposition process.
3.6 SensitivityThe experimental results indicate that the characteristic drop height (H50) of Cu(pn)2(FOX-7)2 is 71 cm (about >14 J). Explosion probability for friction sensitivity is 40 % (25 time experiments). So, Cu(pn)2(FOX-7)2 is relatively less sensitive. Moreover, the impact sensitivity of Cu(pn)2(FOX-7)2 is lower than that of RDX (>7.5 J), but higher than that of FOX-7 (>24.7 J) [30].
4 Conclusions(1) The thermal decomposition of Cu(pn)2(FOX-7)2 exhibits two exothermic processes. The non-isothermal decomposition kinetic equation of the first process is dα/dT=(1017.83/β)3α2/3exp(-1.635×105/RT). The self-accelerating decomposition temperature and critical temperature of thermal explosion are 145.6 and 146.7 ℃, respectively.
(2) Specific heat capacity equation of Cu(pn)2(FOX-7)2 is cp=-2.6824+1.9441×10-2T-2.0494×10-5T2(285.0 K<T<350.0 K), and the molar heat capacity is 653.79 J·mol-1·K-1 at 298.15 K. Adiabatic time-to-explosion of Cu(pn)2(FOX-7)2 is about 77 s. Cu(pn)2(FOX-7)2 is relatively less sensitive (>14 J) (RDX>7.5 J).
| [1] |
Latypov N V, Bergman J, Langlet A, et al. Synthesis and reaction of 1, 1-diamino-2, 2-dinitroethylene[J]. Tetrahedron, 1998, 54: 11525-11536. DOI:10.1016/S0040-4020(98)00673-5 |
| [2] |
Bemm U, Östmark H. 1, 1-Diamino-2, 2-dinitroethylene: a novel energetic material with infinite layers in two dimensions[J]. Acta Crystallographica Section C, 1998, 54: 1997-1999. |
| [3] |
Trzciński W A, Cudzilo S, Chylek Z, et al. Detonation properties and thermal behavior of FOX-7-based explosives[J]. Journal of Energetic Materials, 2008, 31: 72-85. |
| [4] |
Ek S, Ottis J, Dudek K, et al. Scalable synthesis of 1, 1-diamino-2, 2-dinitroethene without hazardous intermediates or by-products[J]. Journal of Energetic Materials, 2013, 31: 87-99. DOI:10.1080/07370652.2011.633964 |
| [5] |
Anniyappan M, Talawar M B, Gore G M, et al. Synthesis, characterization and thermolysis of 1, 1-diamino-2, 2-dinitroethylene(FOX-7) and its salts[J]. Journal of Hazardous Materials, 2006, 137: 812-819. DOI:10.1016/j.jhazmat.2006.03.034 |
| [6] |
Cai H Q, Shu Y J, Yu W F, et al. Research development of 1, 1-diamino-2, 2-dinitroethylene[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2004, 12: 124-128. |
| [7] |
Buszewski B, Michel M, Cudzilo S, et al. High performance liquid chromatography of 1, 1-diamino-2, 2-dinitroethene and some intermediate products of its synthesis[J]. Journal of Hazardous Materials, 2009, 164: 1051-1058. DOI:10.1016/j.jhazmat.2008.09.018 |
| [8] |
Herve G, Guy J, Latypov N. The reactivity of 1, 1-diamino-2, 2-dinitroethene (FOX-7)[J]. Tetrahedron, 2005, 61: 6743-6748. DOI:10.1016/j.tet.2005.05.010 |
| [9] |
Sun Q, Li Y F, Xu K Z, et al. Crystal structure and enthalpy of combustion of AEFOX-7[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2015, 23(12). |
| [10] |
Xu K Z, Song J R, Yang X, et al. Molecular, crystal structure and theoretical calculation and thermal behavior of 2-(1, 1-dinitromethylene)-1, 3-diazepentane[J]. Journal of Molecular Structure, 2008, 891: 340-345. DOI:10.1016/j.molstruc.2008.04.004 |
| [11] |
Xu K Z, Chang C R, Song J R, et al. Preparation, crystal structure and theoretical calculation of G(FOX-7)[J]. Chinese Journal of Chemistry, 2008, 26: 495-499. DOI:10.1002/(ISSN)1614-7065 |
| [12] |
Luo J A, Xu K Z, Wang M, et al. Syntheses and thermal behaviors of Rb(FOX-7)·H2O and Cs(FOX-7)·H2O[J]. Bulletin of the Korean Chemistry Society, 2010, 31(10): 2867-2872. DOI:10.5012/bkcs.2010.31.10.2867 |
| [13] |
Xu K Z, Zuo X G, Song J R, et al. Preparation, crystal structure and thermal behavior of K(FOX-7)·H2O[J]. Chemical Journal of Chinese Universities, 2010, 31: 638-643. |
| [14] |
Chen Y S, Xu K Z, Wang M, et al. A review on reactivity of 1, 1-diamino-2, 2-dinitromethene(FOX-7)[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 120: 120-125. |
| [15] |
Garg S, Gao H X, Parrish D A, et al. FOX-7 (1, 1-diamino-2, 2-dinitroethene): trapped by copper and amines[J]. Inorganic Chemistry, 2011, 50: 390-395. DOI:10.1021/ic102136r |
| [16] |
Vo T T, Parrish D A, Shreeve J M. 1, 1-Diamino-2, 2-dintroethene (FOX-7) in copper and nickel diamine complexes and copper FOX-7[J]. Inorganic Chemistry, 2012, 51: 1963-1968. DOI:10.1021/ic202288t |
| [17] |
He F, Xu K. Z, Zhang H, et al. Two new copper-FOX-7 complexes: synthesis, crystal structure, and thermal behavior[J]. Journal of Coordination Chemistry, 2013, 66(5): 845-855. DOI:10.1080/00958972.2013.771182 |
| [18] |
Yang Q, Chen S P, Xie G. Synthesis and characterization of an energetic compound Cu(Mtta)2(NO3)2 and effect on thermal decomposition of ammonium perchlorate[J]. Journal of Hazardous Materials, 2011, 197: 199-203. DOI:10.1016/j.jhazmat.2011.09.074 |
| [19] |
Stierstorfer J, Tarantik K R, Klapötke T M, et al. New energetic materials: functionalized 1-ethyl-5-aminotetrazoles and 1-ethyl-5-nitriminotetrazoles[J]. Chemistry-A European Journal, 2009, 15: 5775-5792. DOI:10.1002/chem.v15:23 |
| [20] |
Klapötke T M, Stieratorfer J, Weber B. New energetic materials: synthesis and characterization of copper 5-nitriminotetrazolates[J]. Inorganica Chimica Acta, 2009, 362: 2311-2320. DOI:10.1016/j.ica.2008.10.014 |
| [21] |
Gao Z, Huang J, Xu K Z, et al. Synthesis, structural characterization and thermal properties of a new energetic zinc-FOX-7 complex[J]. Journal of Coordintion Chemistry, 2013, 66(20): 3572-3580. DOI:10.1080/00958972.2013.848276 |
| [22] |
Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29: 1702-1706. DOI:10.1021/ac60131a045 |
| [23] |
Ozawa T. A method of analying thermogravimetric data[J]. Bulletin of Chemical Society Jpn, 1965, 38: 1881-1886. DOI:10.1246/bcsj.38.1881 |
| [24] |
Hu R Z, Gao S L, Zhao F Q, et al. Thermal Analysis Kinetics (2th)[M]. Beijing: Science Press, 2008, 151-155.
|
| [25] |
Zhang T L, Hu R Z, Xie Y, et al. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC[J]. Thermochimica Acta, 1994, 244: 171-176. DOI:10.1016/0040-6031(94)80216-5 |
| [26] |
Qiu Q Q, Gao Z, Chen Y S, et al. Non-thermal decomposition kinetics of Cu(NH3)2(FOX-7)2[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(2): 206-209. |
| [27] |
Gao H X, Zhao F Q, Hu R Z, et al. Thermochemical properties, thermal behavior and decomposition mechanism of 1, 1-diamino-2, 2dinitroethylene (DADE)[J]. Chinese Journal of Chemistry, 2006, 24: 177-181. DOI:10.1002/(ISSN)1614-7065 |
| [28] |
Xu K Z, Song J R, Zhao F Q, et al. Thermal behavior, specific heat capacity and adiabatic time-to-explosion of G(FOX-7)[J]. Journal of Hazardous Materials, 2008, 158: 333-339. DOI:10.1016/j.jhazmat.2008.01.077 |
| [29] |
Vyzovkin S, Burnham A K, Criado J M, et al. ICTKA kinetics committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochimica Acta, 2011, 520: 1-19. DOI:10.1016/j.tca.2011.03.034 |
| [30] |
Tian Y D, Zhao F Q, Liu J H. Handbook of Energetic Materials and the Related Compounds[M]. Beijing: National Defense Industry Press, 2011.
|

Thermal decomposition of Cu(pn)2(FOX-7)2 (pn=1, 3-diaminopropane) was studied.




