Over the past decades, considerable efforts have been focused on the development of nitrogen-rich high energy density materials (HEDM) with high performance and decreased sensitivity as well as environmental compatibility[1-3]. One of the most exciting developments in the field of HEDMs is N-heterocyclic energetic compounds with high nitrogen content. Because there are lots of energetic C—N or N—N bonds to enhance the heat of formation and energetic properties. Meanwhile, a mountain of nitrogen were released during the decomposition process which makes them have a wide use in gas generating agent, low characteristic signal propellant, high energy yet low sensitivity materials[4-6]. 3, 7-Bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane (BNITABO) is a homothetic energetic compound of this type containing two nitroimino groups and a large number of C—N or N—N bonds in the molecule. Though, BNITABO has been synthesized, little research was found on its thermal behavior, electronic structure and energetic properties (heats of formation, detonation velocity and detonation pressure)[7]. In the presented paper, the thermal properties, electronic structure and detonation performances of 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane were fully investigated.
2 Experiments 2.1 MaterialAccording to Ref [8], 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane (Scheme 1, C4H6O4N8) was prepared though addition, condensation and nitration reactions. Yield 58%, m.p. 326 ℃, 1H NMR (DMSO-d6, 500 MHz) δ: 9.53 (s, 4H), 5.84 (s, 2H); 13C NMR (DMSO-d6, 125 MHz) δ: 161.9, 69.6; IR (KBr, ν/cm-1): 3190, 1504, 1558, 1435, 1270, 1200, 1071, 1045, 1017, 952, 884, 780, 753, 637; m/z(%): 229 (M-, 100); ρ, 1.87 g·cm-3.
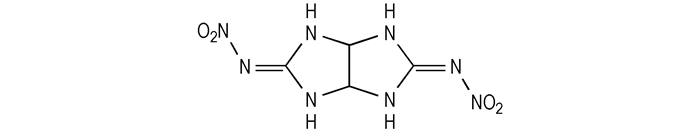
|
Scheme1 Structure of 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane |
1H NMR, 13C NMR, IR, MS spectrum and computations were performed on the Bruker Avance Ⅲ 500 MHz, Thermo Nicolte IS10 IR instrument, Finnigan TSQ Quantum Mass Spectrometer and Gaussian 03 program with the B3LYP/6-31G(d, p) basis set[9-10], respectively. TG-DTG-DSC curves were also performed on a NETZSCH STA 409 PC/PG coupling system with an initial mass of about 3.0 mg placed in alumina crucibles (nitrogen atmosphere with the flow rate of 30 mL·min-1).
3 Results andDiscussion 3.1 Thermal propertiesFig. 1 shows the TG-DTG-DSC curves of BNITABO at the heating rate of 5 K·min-1. From the Fig. 1, it is seen that the decomposition of the title compound is a two-steps process. The first step starts from 280 ℃ to 320 ℃ with 60% mass loss and the second step starts from 320 ℃ to 650 ℃ with about 40% mass loss. Correspondingly, there is an evident sharp peak at about 320 ℃ and a faint peak at around 550 ℃ in the DTG curve.
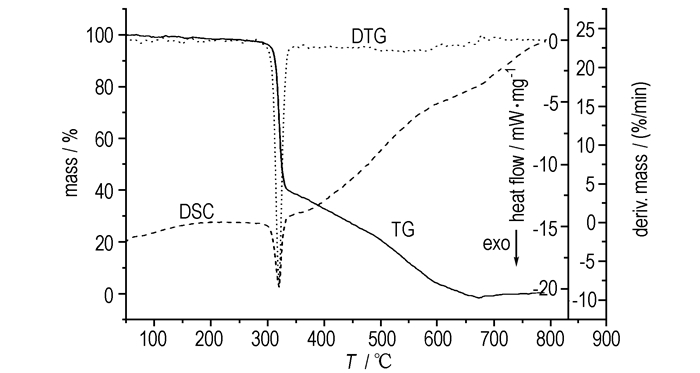
|
Fig.1 TG-DTG-DSC curves of BNITABO at 5 K·min-1 |
Fig. 2 shows the TG-DSC curves of the decomposition process of BNITABO at different heating rates of 5, 10, 15 K·min-1 and 20 K·min-1. It was found that the decomposition temperatures and the exothermic peaks shifted to higher temperatures as the heating rate increased. This may be attributed to the "thermal hysteresis" of the heat transfer effect at different heating rates.
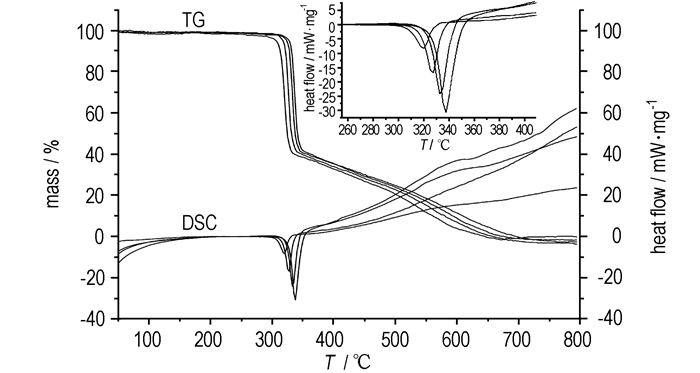
|
Fig.2 TG-DSC curves of BNITABO at different heating rates |
The relative kinetic parameters such as activation energy (Ea), pre-exponential factor (A), entropy of activation (ΔS≠), enthalpy of activation (ΔH≠), free energy of activation (ΔG≠) and the critical ignition temperature (Tb) were obtained based on the following methods.
(1) Kissinger equation[11]:
| $ {\rm{ln}}\frac{{{\beta _{\rm{i}}}}}{{T_{{\rm{pi}}}^2}} = {\rm{ln}}\frac{{{A_{\rm{K}}}R}}{{{E_{\rm{K}}}}}-\frac{{{E_{\rm{K}}}}}{{R{T_{{\rm{pi}}}}}} $ | (1) |
(2) Ozawa-Flynn-Wall equation[12]:
| $ {\rm{lg}}\beta = C-0.4567\frac{{{E_O}}}{{RT}} $ | (2) |
where, β is the heating rate, K; Tpi is the maximum peak temperature which can be obtained from the DSC curves, K; R is the gas constant, EK and EO are the activation energy calculated by the Kissinger and Ozawa′s methods, kJ·mol-1, respectively; A is the pre-exponential factor, C is the constant.
β, Tpi and the kinetic parameters obtained by Kissinger and Ozawa methods were summarized in Table 1. It is obviously seen that the values of Tpi of the exothermic peak shifted to higher temperatures due to the "thermal hysteresis" of the heat transfer effect. On the other hand, the values of E calculated by Kissinger method (EK=225.80 kJ·mol-1) very similar with that obtained by Ozawa's method (EO=224.24 kJ·mol-1). Besides, both of the linear correlation coefficients (rK=0.9945 and rO=0.9949) are very close to 1. All the above-obtained data indicates that the results are credible.
| Tab.1 Calculated values of the kinetic parameters of title compound |
Then, the values of ΔS≠, ΔH≠, ΔG≠ and Tb can be obtained based on the following equations combined with the calculated EK and A[13].
| $ A{\rm{exp}}\left( {-\frac{E}{{RT}}} \right) = \frac{{{k_{\rm{B}}}T}}{h}{\rm{exp}}\left( {\frac{{\mathit{\Delta }{S^ \ne }}}{R}} \right){\rm{exp}}\left( {-\frac{{\mathit{\Delta }{H^ \ne }}}{{RT}}} \right) $ | (3) |
| $ \Delta {H^ \ne } = {E_{\rm{a}}}-RT $ | (4) |
| $ \Delta {G^ \ne } = \Delta {H^ \ne }-T\Delta {S^ \ne } $ | (5) |
Where, T=Tp0, the peak temperature (Tpi) corresponding to β→0;Ea=EK, calculated by Kissinger′s method; A=AK, calculated by Kissinger′s method; ΔS≠, ΔH≠ and ΔG≠ are the entropy of activation, the enthalpy of activation and the free energy of activation, respectively; kB is the Boltzmann constant, 1.3807×10-23 J·K-1; h is the Plank constant, 6.626×10-34 J·s.
Tp0 could be calculated by Eq.(6).
| $ {T_{{\rm{pi}}}} = {T_{{\rm{p0}}}} + b{\beta _i} + c\beta _i^2 + d\beta _i^3 $ | (6) |
Where, b, c and d are coefficients.
Based on the above-described equations, the value of Tp0, ΔS≠, ΔH≠, and ΔG≠ were calculated as 586.85 K, 80.18 J·mol-1·K-1, 220.92 kJ·mol-1 and 173.87 kJ·mol-1, respectively.
The critical temperature of thermal explosion, as an important parameter during the storage or usage of an energetic material, was calculated by Eq.(7).
| $ {T_b} = \frac{{{E_{\rm{O}}}-\sqrt {E_{\rm{O}}^2-4{E_{\rm{O}}}R{T_{{\rm{p0}}}}} }}{{2R}} $ | (7) |
Where, Tb is the critical temperature of thermal explosion; EO is the apparent activation energy obtained by Ozawa′s method; R is the gas constant; Tp0 is the peak temperature corresponding to β→0.
The calculated value of Tb is 600.25 K, which indicates that BNITABO satisfy the meets of the safety.
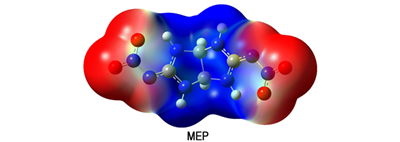
3.3 Electronic structureMolecular orbital and the electronic structure of BNITABO were investigated based on the B3LYP/6-31G(d, p) level-optimized structure. The calculated energy gap value of BNITABO is 5.58 eV, indicating it may have a lower reactivity. Fig. 3 illustrates the 3D plots of the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO) and the molecular electrostatic potentials (MEP) of BNITABO. There exists 59 the highest occupied molecular orbital and 60 the lowest unoccupied molecular orbital in the molecule. It is also obviously seen that most of the HOMO and LUMO levels are 2-fold degenerate indicating that the removal of an electronic from the HOMO level or addition of an electronic to the LUMO level could weaken the skeleton framework. In view of the MEP, the negative potentials appear to be distributed mostly on the oxygen atoms of the —NO2 groups while the positive potentials appear to be at the center of the skeleton. It may attribute to the stabilization of the molecular structure according to the law proposed by Klapotke et al[14].

|
Fig.3 HOMO, LUMO and MEP of 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane |
Detonation performances are of great importance parameter for an energetic material and could be obtained according to Kamlet-Jacobs equations[15].
| $ D = 1.01{(N{{\bar M}^{0.5}}{Q^{0.5}})^{0.5}}(1 + 1.3\rho ) $ | (8) |
| $ P = 1.558{\rho ^2}N{{\bar M}^{0.5}}{Q^{0.5}} $ | (9) |
Where, ρ is the crystal density of the explosive, g·cm-3; D is the detonation velocity, km·s-1; p is the detonation pressure, GPa; N is the number of moles of detonation gases per-gram explosive, mol·g-1; M is the average molecular weight of these gases, g·mol-1; and Q is the heat of detonation, J·g-1.
The accurate heat of formation of BNITABO can be calculated using the isodesmic reaction[16].

|
Scheme2 Isodesmic reaction designed for the title compound |
Based on the above-described equations, the accurate heat of formation of BNITABO was obtained as 514.8 kJ·mol-1. In view of the detonation velocities (8.70 km·s-1, ρ=1.87 g·cm-3) and detonation pressure (34.34 GPa), its detonation performances are superior to those of TNT(D=6.8 km·s-1; p=19.5 GPa)[17], and similar with those of RDX (D=8.75 km·s-1; p=34.0 GPa)[18], indicating that BNITABO can be used as a potential candidate of high-energy and stable energetic material.
4 Conclusions(1) The thermaldecomposition of 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane presents a two-stage process with the mass loss of 100% and a single sharp exothermic peak were observed at around 320 ℃.
(2) The calculated Ea via Kissinger and Ozawa methods are 225.80 and 224.24 kJ·mol-1, respectively. Thermodynamic parameters such as ΔS≠, ΔH≠, G≠ and Tb were calculated as 80.81 J·mol-1·k-1, 220.92 kJ·mol-1, 173.87 kJ·mol-1 and 600.25 K, respectively.
(3) The electron structure of 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane shows that the negative potentials appear to be distributed—NO2 groups and the positive potentials appear to be at the center of the skeleton which attribute to the stabilization of the molecular structure.
(4) The calculated values of detonation velocity (D=8.70 km·s-1) and detonation pressure (p=34.34 GPa) involve that 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane has the potential to be used as high-energy and stable energetic material.
| [1] |
Pagoria P F, Lee G S, Mitchell A R, et al. A review of energetic materials synthesis[J]. Thermochimica Acta, 2002, 384(1): 187-204. |
| [2] |
JIN Xing-hui, HU Bing-cheng, LU Wei, et al. Theoretical study on a novel high-energy density material 4, 6, 10, 12-tetranitro-5, 11-bis (nitroimino)-2, 8-dioxa-4, 6, 10, 12-tetraaza-tricyclo[7, 3, 0, 03, 7]dodecane[J]. RSC Advances, 2014, 4(13): 6471-6477. DOI:10.1039/c3ra46107f |
| [3] |
WEI Zhen, LI Jia-rong, ZHANG Qi, et al. Review on diamondoids as high energetic fuels[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(2): 170-176. |
| [4] |
Rahm M, Dvinskikh S V, Furo I, et al. Experimental detection of trinitramide N(NO2)3[J]. Angewandte Chemie, 2011, 123(5): 1177-1180. DOI:10.1002/ange.v123.5 |
| [5] |
JIN Xing-hui, HU Bing-cheng, LIU Zu-liang, et al. One-pot synthesis of 2, 4, 5-trinitroimidazole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(5): 722-724. |
| [6] |
JIA Huan-qing, HU Bing-cheng, JIN Xing-hui. Synthesis and Thermal Properties of 1, 2-Dinitroguanidine[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(5): 722-724. |
| [7] |
JIN Xing-hui, HU Bing-cheng, LIU Zu-liang. Thermal stability and quantum chemistry study on octahydro-2, 5-bis (nitroimino) imidazo[4, 5-d]imidazole[J]. Procedia Engineering, 2012, 45: 558-561. DOI:10.1016/j.proeng.2012.08.203 |
| [8] |
Kony M, Dagley I J. Synthesis of octahydro-2, 5-bis (nitroimino)imidazo[4, 5-d]imidazole[J]. Heterocycles, 1994, 38(3): 595-600. DOI:10.3987/COM-93-6597 |
| [9] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 03, 2003, Gaussian[CP]. Inc. : Pittsburgh, PA.
|
| [10] |
Flippen-Anderson J L, Kony M, Dagley I J. Acta Crystallographica Section C, 1994, 50(4), 974-976[J]. Acta Crystallographica Section C, 1994, 50(4): 974-976. |
| [11] |
Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical chemistry, 1957, 29(11): 1702-1706. DOI:10.1021/ac60131a045 |
| [12] |
Ozawa T. A new method of analyzing thermogravimetric data[J]. Bulletin of the chemical society of Japan, 1965, 38(11): 1881-1886. DOI:10.1246/bcsj.38.1881 |
| [13] |
Criado J M, Perez-Maqueda L A, Sanchez-Jimenez P E. Dependence of the preexponential factor on temperature[J]. Journal of thermal analysis and calorimetry, 2005, 82(3): 671-675. DOI:10.1007/s10973-005-0948-3 |
| [14] |
Hammerl A, Klapotke T M, Nöth H, et al. Synthesis, structure, molecular orbital and valence bond calculations for tetrazole azide, CHN7[J]. Propellants, Explosives, Pyrotechnics, 2003, 28(4): 165-173. DOI:10.1002/(ISSN)1521-4087 |
| [15] |
Kamlet M J, Jacobs S J. Chemistry of detonations. I. A simple method for calculating detonation performances of C—H—N—O explosives[J]. The Journal of Chemical Physics, 1968, 48(1): 23-35. DOI:10.1063/1.1667908 |
| [16] |
JIN Xing-hui, HU Bing-cheng, JIA Huan-qing, et al. DFT Theoretical Study on Energetic Nitrogen-Rich Derivatives of C4N6H8-n(NO2)n[J]. Quim Nova, 2014, 37(1): 74-80. DOI:10.1590/S0100-40422014000100014 |
| [17] |
Zhang Q H, Zhang J H, Parrish D A, Shreeve J M. Energetic N-trinitroethyl-substituted mono-, di-, and triaminotetrazoles[J]. Chemistry-A European Journal, 2013, 19(33): 11000-11006. DOI:10.1002/chem.201300994 |
| [18] |
Talawar M B, Sivabalan R, Mukundan T, et al. Environmentally compatible next generation green energetic materials (GEMs)[J]. Journal of Hazardous Materials, 2009, 161(2): 589-607. |
The thermal properties, the electronic structure and detonation properties of a nitrogen-rich energetic material 3, 7-bis(nitroimino)-2, 4, 6, 8-tetraazabicyclo[3.3.0]octane were investigated by the TG-DTG-DSC mothed and the quantum chemical method, respectively. It can be used as a potential candidate of high-energy and stable energetic material.




