The metal hydride was a fine hydrogen-storage material whose mass contents had reached 5%-15% and the volume hydrogen density was twice higher than that of the liquid hydrogen[1-4]. Due to the low average molecular mass of burning gas, the metal hydride was an additive which was suitable for the solid propellant. As the high-efficiency hydride storage material, the thermal decomposition temperature was between 100 ℃ to 900℃, which was lower compared to the burning temperature of propellant in the combustor. In the combustion, the hydride was released and combined with metal which was separated from the metal hydride. The mixture of hydrogen and metal was burned and released energies, so that the augment of the energy reaction system lead to the specific impulse to increase.
The research on metal hydride materials and the application of them in the energetic materials had been studied systematically in the world. Tskhai A.N.[5] studied the thermal decomposition of AlH3 and AlD3 by NMR and the results showed that the decomposition rate of AlD3 was half of that of AlH3. Flynn[6] added AlH3 into the double-base propellant and the AlH3 showed excellent performance. The theory impulse of propellant improved to 2874.34 N·s·kg-1 and the measured specific impulse reached 2675.4 N·s·kg-1. The Signal Processing laboratory [7] studied the burning rate and ballistic performances of AlH3 high energy fuel at 0.1-7 MPa. The results showed that the stable burning rate and surface temperature of AlH3 formula were higher than that of Al formula. With the mass contents increasing, the pressure index decreased and propellant ignited easily. The research of propellant containing metal hydride was at an entry-level, especially in the part of energy performance, it could not provide the absolute assumption about design of propellant formula. Gui Dayong[8] had studied the influence of NaBH4 which was added into the Ba(NO3)2/RP/Mg incendiary agent system and the result of the system indicated that the NaBH4 could improve the heat of incendiary agent and decreased the sensitivity at the same time. Liu Leili[9] has researched how the Mg2NiH4、Mg2Cu—H and MgH2 effect the decomposition of ammonium perchlorate and added the 3 hydrogen storage materials into AP/Al/HTPB propellant as catalyst. The consequences suggested that they could all increase the burning rate and the effects of Mg2Cu—H and MgH2 were better. Li Meng[10]had made the research on the effect of metal hydride on the heat properties of composite propellant and discovered that the standard theoretical specific impulse of the composite propellant increased by 3.2%, 1.13% and 0.7%. All of them had larger contributions than Al to the standard theoretical specific impulse. Pei Jiangfeng[11] calculated the energy properties of LiAlH4-p(BAMO-AMMO) and Mg(AlH4)2-p(BAMO-AMMO) propellants, and when LiAlH4 and Mg(AlH4)2 replaced Al powder, the energy of propellants could be improved.
Due to the instability and consistency in propellant, the method was designed to passivate the metal hydride materials in the study. Paraffin and Naphthalene were chosen as surface additives to coat the KBH4、NaBH4 and LiAlH4 to improve the stability. The coated condition was investigated by SEM、FTIR and XRD. The burning characteristics were tested by an Infrared Thermometer.
2 Experimental 2.1 Experimental Raw MaterialTable 1 shows the experimental raw materials.
| Tab.1 The experimental raw materials |
The dimethylbenzene solution of paraffin was prepared with 5 g paraffin dissolved in 100 g dimethylbenzene. And then the NaBH4(3 g) were put into beaker and stirred for 30 min on a magnetic stirrer until the NaBH4 dissolved in the solution equably. And then put the mixed liquor into isopropyl alcohol slowly and stir until the paraffin separated out invariantly. Lastly, filter the solution and dry the sample at 50 ℃ for 2 h.
2.2.2 Coating Process of KBH4The dimethylbenzene solution of paraffin was prepared with 5 g paraffin dissolved in 100 g dimethylbenzene. And then the KBH4(3 g) were put into beaker and stirred for 30 min on a magneticstirrer until the KBH4 dissolved in the solution equably. And then put the mixed liquor into methyl alcohol slowly and stir until the paraffin separated out invariantly. Lastly, filter the solution and dry the sample at 50 ℃ for 2 h.
2.2.3 Coating Process of LiAlH4Napthalene was dissolved in dimethylbenzene to prepared supersaturated solution at 60 ℃, And then add 3 g LiAlH4 into the supersaturated solution and stir for 30 min to dissolve the LiAlH4. After that, stop the heating to make the sample crystallize at the room temperature. Filter the mixed liquor and place the filters at -10 ℃ for 6 h at least. The dry coated LiAlH4 was prepared successfully.
2.2.4 Preparation Process of PropellantTable 2 shows the formula and the physical parameter of propellants and Fig. 1 is the preparation process of propellants.
| Tab.2 The formula and the physical parameter of propellants |
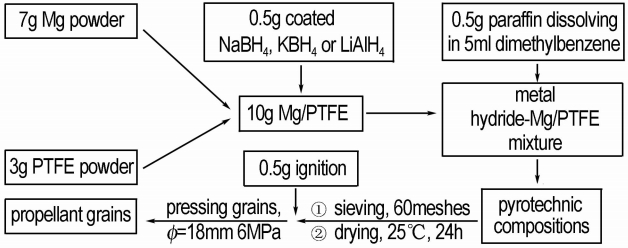
|
Fig.1 The preparation process of propellant |
For this work, the powder samples were prepared with the chemicals of composites of 7.0 g Mg and 3.0 g PTFE, and the additive always being 0.5 g NaBH4, KBH4 or LiAlH4. Secondly the metal hydride-Mg/PTFE was added in the solution that 0.5 g paraffin was dissolved in 5 mL dimethybenzene. The wet mash should be sieved in griddle which was 60 meshes and dried for 24 h at 25 ℃. Thirdly, put the dry compositions and 0.5 g ignition into the mould (Ф=18 mm) and press the powders under 6 MPa. The propellant grains were prepared successfully.
2.3 Test InstrumentsThe morphologies of coated metal hydride were observedwith scanning electron microscopy (JEOLJSM-6380LV, JEOL).
The crystalline structures of the synthesized product were identified with a powder X-ray diffraction (Germany Bruker Corporation), employing CuKα radiation (0.15406 nm), with scanning speed 5°/s and 2θ:10°-80°.
The surface mass was tested bya Fourier transform infrared spectrometer (Nicolet IS-10, ThermoFisher).
The burning temperature was testedwith an Infrared Thermometer (IGA 140, Germany IMPAC Instrument Corporation) whose measurement range was 340-2500 ℃. The probe should aim at the propellant grain and the distance was 2 m.
3 Results and Discussions 3.1 Characterizations of the Coated Metal HydrideThe microstructure of the coating layers are investigated by SEM and the graphs of results are as follows. Fig. 2-Fig. 4 are the images of NaBH4 and KBH4 coated by paraffin and LiAlH4 coated by naphthalene. Fig. 2 shows that there is a thin film covering on the NaBH4 particles. The layer of paraffin is distributed uniformly in the picture that is magnified 2000 times. There are a few smooth parts on the surface of the layer. The smooth parts are lamellar in the amplification of 5000 times and 10000 times. The test results show that the NaBH4 particles are coated completely. Fig. 3 shows the coating situations of KBH4. Fig. 3a and Fig. 3b appear three lamellas on the particle and the surface of the layer is uniform. But Fig. 3c displays the boundary of the lamella and it is clear that the structure of paraffin on the particles has many stratums, so the KBH4 is coated perfectly. Fig. 4 is the SEM pictures of LiAlH4 particle that is coated by naphthalene. The Fig. 4a shows the LiAlH4 and naphthalene stacks confusedly. The surface of naphthalene is not smooth and the structure of naphthalene has many layers in Fig. 4b and Fig. 4c. The pictures prove the LiAlH4 has a worse covered effect. The coating effect of paraffin on the NaBH4 and KBH4 prepared by solvent-nonsolvent method is better because the solubility of paraffin in dimethylbenzene and isopropyl alcohol has great differences and the paraffin is amorphousbody. Naphthalene is crystal and can form aggregation easily when it is recrystallized with the solution cooled, so the coated shell is irregular.
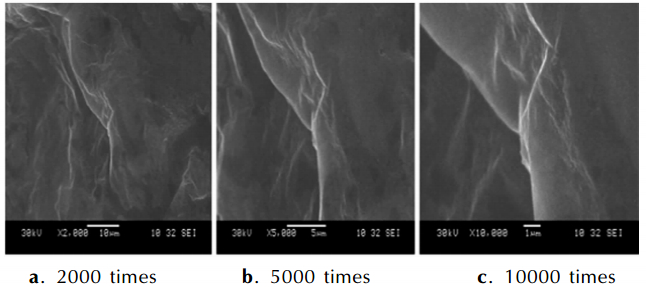
|
Fig.2 SEM images of NaBH4 coated by paraffin in different amplification |
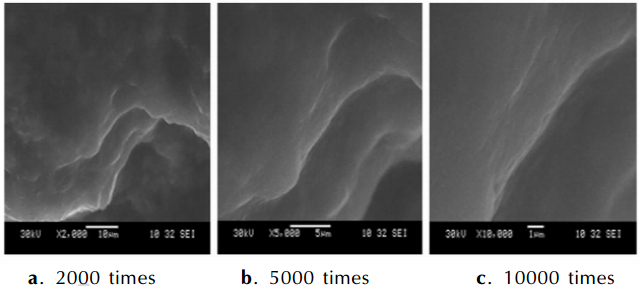
|
Fig.3 SEM images of KBH4 coated by paraffin in different amplification |
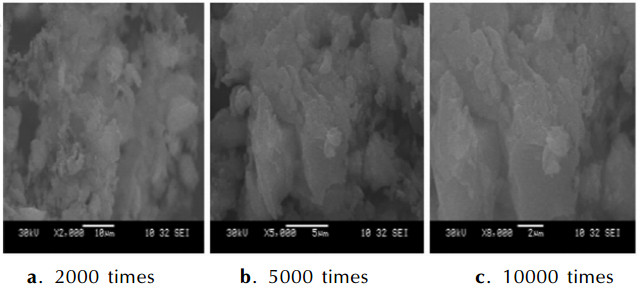
|
Fig.4 SEM images of LiAlH4 coated by naphthalene in different amplification |
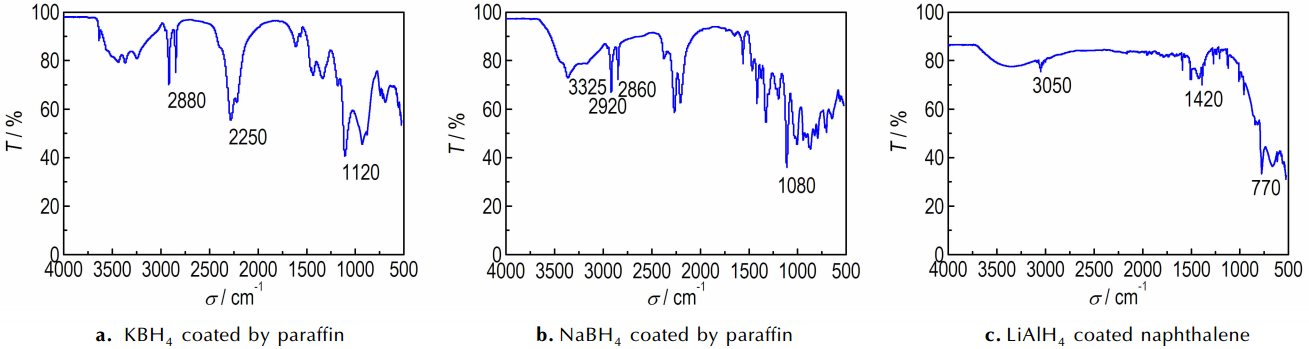
Fig. 5 shows the infrared spectrum absorption characterization of coating layers to determine the components through infrared spectrometer. In Fig. 5a, the infrared absorption spectrum shows a weak and wide absorption peak that is —O—H stretching vibration band at 3325 cm-1 between 3500-3250 cm-1. There are two stretching vibration absorption peaks of C—H(sp3) at 2920 cm-1 and 2860 cm-1. There exist two absorptions at 2250-1cm and both of them are the stretching vibration of nitrile. The peak near 1465-1340 cm-1 is attributable to the bending stretching of C—H. There is a strong absorption peak (1080 cm-1), corresponding to the stretching vibration absorption spectra of C—O. The results show that the coating layers contain paraffin and primary alcohol so that the coating samples have paraffin and methyl alcohol. There is a weak and wide absorption peak between 3500-3250 cm-1 and it is the stretching vibration peak of —O—H in Fig. 5b. Strong stretching vibration absorption peak of C—H(sp3) appears at 2880 cm-1. There is a medium absorption peak at 2250 cm-1 and it is the stretching vibration of nitrile. The bending vibration absorption peak of C—H exists at 1465-1340 cm-1. The stretching vibration absorption peak of C—O is at 1120 cm-1. The FTIR proves the existence of paraffin and isopropyl alcohol. Fig. 5c shows a stretching vibration absorption peak of C—H of benzene ring at 3050 cm-1. There is a vibration absorption peak of C=C of benzene ring at 1600-1450 cm-1. The out of plane bending vibration absorption peak of C—H is at 770 cm-1 between 880-680 cm-1. In conclusion, the coated LiAlH4 sample has aromatic hydrocarbon so that we can confirm the material is naphthalene.
|
Fig.5 Infrared spectra of different samples |
The X-ray diffraction analysis images of coated samples through X-ray diffraction are as follows.
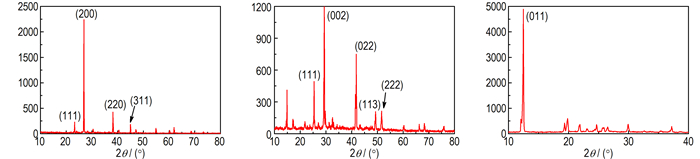
Fig. 6a shows the X-ray diffraction of KBH4 coated by paraffin and there are 4 diffraction peaks on the image. The XRD pattern (2θ=22.884°, 26.458°, 37.784°, 44.622°) is compared with JCPDS10-0112(KBH4), which corresponds to the crystal facet parameter of (111), (200), (220) and (311). Because the paraffin layer is thin and the paraffin is non-crystal, there is no diffraction of paraffin. Fig. 6b displays the small angle XRD patterns of NaBH4 that is coated by paraffin. Here are 4 peaks of NaBH4 on the picture and the XRD pattern (2θ=25.124°, 29.105°, 41.637°, 49.190°) is compared with JCPDS38-1022(NaBH4). The samples exhibit diffraction peaks that can be indexed to the (111), (002), (113) and (222) diffractions being characteristic of the NaBH4. This indicates that the NaBH4 is not destroyed and the coating layer is thin and complete. The XRD pattern for the samples of LiAlH4 coated by naphthalene is presented in Fig. 6c. The XRD pattern (2θ=12.114°) mainly contain peaks of LiAlH4 and it is compared with JCPDS12-0473(LiAlH4), which corresponds to the crystal facet parameter of (011).But there is no diffraction peak of naphthalene in the picture because the LiAlH4 is not coated well and some LiAlH4 is exposed in the air.

|
Fig.6 XRD images of different samples |
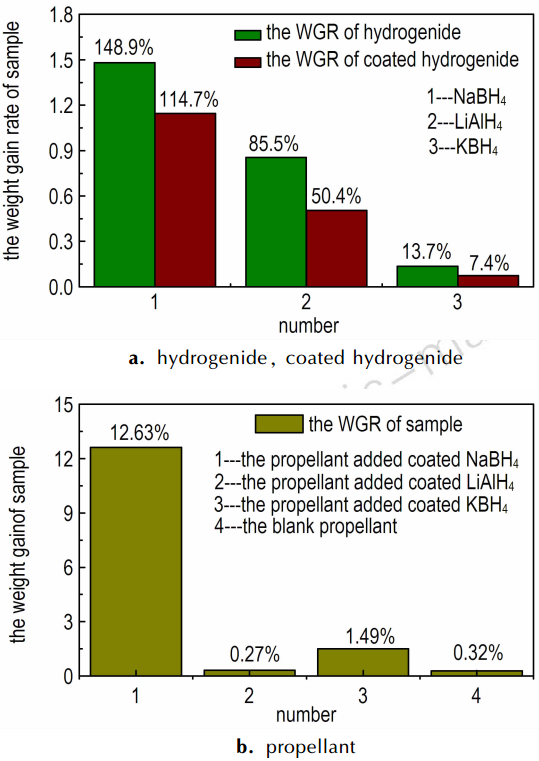
Fig. 7 is the pictures of the storage performances of hydrogenide, coating hydrogenide, propellant and propellant added coated hydrogenide. The data was tested following GJB 5382.7-2005.
|
Fig.7 Weight gain rate of different samples |
Fig. 7a is the picture of the weight gain rate(WGR) of hydrogenide compared with coated hydrogenide. The WGR of hydrogenide is higher than those of coating hydrogenide. The NaBH4 and LiAlH4 have strong power of water absorption so that the weight gain rate has reached 148.9% and 85.5%, but the rate of KBH4 has only gained 13.7%. The hydrogenide which is coated a layer of paraffin or naphthalene has a lower hygroscopicity. The WGR of coated NaBH4, LiAlH4 and KBH4 is 114.7%, 50.4% and 7.4% respectively. The decreased degree is obvious. Fig. 7b is the photo of the WGR of blank propellant compared with the propellant added coated NaBH4, coated LiAlH4 and coated KBH4.The propellant added coated NaBH4 has gained 12.6% and the KBH4 group has gained 1.49%.The data are all higher than that of the blank propellant which has only increased 0.32%.But the LiAlH4 group gains 0.27% which is lower than that of the blank group because the naphthalene has volatility. If the storage time was long, the naphthalene will volatilize completely which will lead to the LiAlH4 exposuring to the air. The LiAlH4 will lose efficacy because it can absorb the vapor and react with water. From the above, the storage property of coated KBH4 group was best.
3.5 The Combustion Properties of PropellantsIn order to estimate the effect of coating activated metal hydride in the propellant compared with control group, the coating activated metal hydride are added into the Mg/PTFE propellant separately. Table 3 shows the combustion data of propellant. Groups 1, 2, 3 are the propellants groups (KBH4-Mg/PTFE, NaBH4-Mg/PTFE and LiAlH4-Mg/PTFE) and group 4 is the control group (Mg/PTFE). The operating pressure is the ordinary pressure (101.325 kPa).
| Tab.3 Combustion properties of propellants |
Table 3 shows the combustion performances of the propellants which add the additives into the Mg/PTFE basic formula. The highest average data of the maximum combustion temperature is the group that the grains are added NaBH4 and it is 40.8 ℃ higher than that of the control group. The mean combustion temperature of NaBH4 group is also the highest and it is 41.2 ℃ higher than that of the control group. The average data of the maximum combustion temperature of group1 is 31.9 ℃ higher than the control group, but the mean combustion temperature is almost the same as the controls. The maximum and mean combustion temperatures of LiAlH4 group are both lower than that of controls. The data of the former is 43.4 ℃ lower than control group and the latter is 67.1 ℃.The mean mass burning rates of propellants contained NaBH4 and KBH4 increase 0.019 g·cm-2·s-1 and 0.02 g·cm-2·s-1, respectively. The increased percentages are 7.72% and 7.34% compared with the control group. The mean burning rates of NaBH4 and KBH4 groups improve 0.1 mm·s-1 and 0.13 mm·s-1. Meanwhile, the increased percentages have reached 5.88% and 7.65%. But the mass burning rate of LiAlH4 group decreases about 20.8% and the burning rate reduces 21.1%. The combustion temperature of Groups 1 and 2 is higher than the controls due to the higher mass burning rate which leads to the energy released quickly in a short time. It can also explain the reason that the temperature data of Group 3 is lower than control group. The cause of the lower burning rate of LiAlH4 group is probably LiAlH4 coated by naphthalene needs to absorb more heat than the KBH4/NaBH4 coated by paraffin.
4 ConclusionsThe NaBH4 and KBH4 are coated by paraffin via the solvent-nonsolvent method successfully. The LiAlH4 is coated by naphthalene via recrystallization method. The NaBH4 and KBH4 are coated best and the layers are compact and uniform, but the coating layer of LiAlH4 is rough. The propellant of adding the coated KBH4 has the best storage property in three groups. The combustion temperature of propellant added coated NaBH4/KBH4 is higher than that of control group, but the LiAlH4 group is lower than that of control group. The mass burning rate and line burning rate of NaBH4 group improve by 7.3% and 5.9% and those of KBH4 group increase by 7.7% and 7.6%. But those of the LiAlH4 group decrease by 20.8% and 21.2% respectively. In three groups, the KBH4 group has the best comprehensive performance.
| [1] |
Guery J F, Chang Is. Solid propulsion for space applications: an updated road map[J]. Acta Astronautics, 2010, 66: 201-219. DOI:10.1016/j.actaastro.2009.05.028 |
| [2] |
Bazyn T, Eyer R, Kirer H, et al. Dehydrogenation and burning of aluminum hydride at elevated pressures[C]//42nd AIAA Aerospace Sciences Meeting. Reno, NV, 2004.
|
| [3] |
Wojciech G, Peter P E. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen[J]. Chemical Reveiws, 2004, 104: 1283-1315. DOI:10.1021/cr030691s |
| [4] |
Javaid A, Mrinal G, Pundalik S D, et al. Nanocomposites: An ideal coating material to reduce the sensitivity of hydrazinium nitroformate[J]. Propellants, Explosives, Pyrotechnics, 2010, 35(2): 153-158. DOI:10.1002/prep.200900024 |
| [5] |
Takhai A N, Zakharov V V, Nechiporenko G N, et al. The molecular association of aluminium hydride in ethers[J]. Russian Journal of Inorganic Chemistry, 1992, 37(9): 997. |
| [6] |
Flynn J P, Lane G A, Plomer J J. Nitrocellulose propellant composition containing aluminium hydride: US, 3844895[P], 1965.
|
| [7] |
CHEN Zhi-xia, ZHENG Gan-yong, WANG Yong-chang, et al. Research on new energetic material aluminum hydride applied in hydro reactive metal fuel[J]. Ship Science and Technology, 2010, 32(12): 24-27. |
| [8] |
GUI Da-yong, LIU Ji-ping, DAI Lan. Application of sodium borohydride(NaBH4) in incendiary agent[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2007, 15: 231-234. |
| [9] |
LIU Lei-li, LI Feng-sheng, ZHI Chun-lei, et al. Effect of magesium based hydrogen storage materials on the properties of AP/Al/HTPB composite solid propellant[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2009, 17(5): 501-504. |
| [10] |
LI Meng, ZHAO Feng-qi, XU Si-yu, et al. Energetic characteristics of composite propellant containing different metal hydride[J]. Journal of Solid Rocket Technology, 2014, 37(1): 86-90. |
| [11] |
PEI Jiang-feng, ZHAO Feng-qi, JIAO Jian-she, et al. Energetic characteristics of BAMO-AMMO based propellants containing differentmetal hydride[J]. Explosive Materials, 2014, 43(4): 11-15. |
KBH4 and NaBH4 were coated by paraffin via solvent-nonsolvent method and LiAlH4 was coated by naphthalene via recrystallization. The surface coating situation of the samples was studied by SEM, FTIR and XRD and the combustion properties of the propellants added into coated sample were tested by an infrared thermometer.



