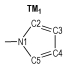
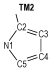
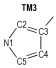
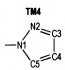
Due to the high nitrogen contents and large positive enthalpies of formation, azoles and their derivatives are an important and interesting topic in the field of study of high energetic materials, and are considered as potential propellants and explosives. Huynh[1] synthesized one ditetrazole derivative from 3, 6-diazido-1, 2, 4, 5-tetrazine. Abe [2] successfully synthesized 5-azido-(1-dialkylimino)-tetrazole. 1, 5-Diamino-4-methyl-tetrazolium dinitramide was synthesized by a metathetical reaction of the corresponding iodide and silver dinitramide[3]. Some derivatives of 4, 4′-bis(5-nitro-1, 2, 3-2H-triazole) were designed, synthesized, and characterized by He[4]. Comparing to the most of current-used propellants, they produced less CO and CO2, when combustion, which meant more environmental-friendly and might decrease the gun barrel and the rocket nozzle erosion. Among the derivates of azoles, the azo-bridged azoles had been extensively studied recent years, because the azo group could increase the enthalpy of formation remarkably, as an important factor of detonation properties[5-9]. When the azo group attached to the nitrogen atoms of azoles, a long chain of nitrogen would be formed. For example 1, 1′-azobis(1, 2, 3-triazole)[10] and 1, 1′-azobis(1, 2, 3, 4-tetrazole)[11], which have been reported and present excellent property, contained nitrogen chains consisted of 8 and 10 nitrogen atoms respectively.
The experimental experiences indicated that the nitrogen content, position of nitrogen atoms and decomposition mode of azo-bridged azoles could affect their thermochemical properties remarkably. However, to our knowledge, the thermochemical properties of azo-bridged azoles had never been well-studied by theoretical calculation. To uncover the relationships between their structures and thermochemical properties, we examined the structures and thermochemistries of 15 azo-bridged azoles using DFT method at B3LYP/6-311+G(d, p) level in this work.
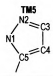
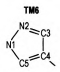
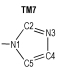
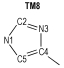
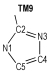
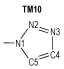
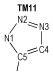
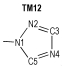
2 Computational MethodsThe structural formulas of azo-bridged azoles are illustrated in Scheme 1.
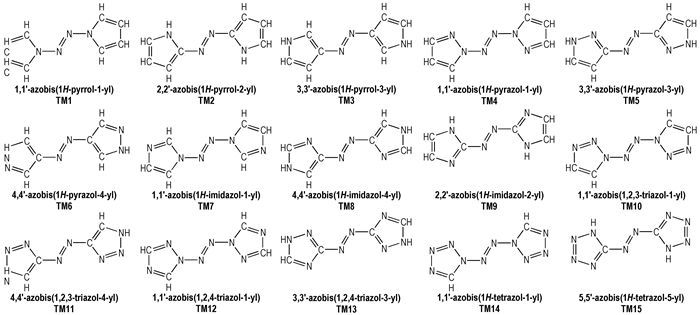
|
Scheme1 Structural formulas of the azo-bridged azoles |
All calculations were performed with the Gaussian 09 suite[12]. B3LYP density functional theoretical method with 6-311+G(d, p) basis set was selected for the geometry optimization and frequency calculation because it provides greater account of electron correlation.
Standard enthalpy of formation (ΔfH298Θ) was calculated using the isodesmic reaction, which featured same number and type of bonds on each side of the reaction but often lead to cancellation by errors from the bond environments.
For the isodesmic reaction, the enthalpy of formation at 298 K was obtained from the following equation
| $ {\Delta _{\rm{r}}}H = \sum {\Delta {H_{{\rm{f,p}}}} - \sum {\Delta {H_{{\rm{f,R}}}}} } $ | (1) |
Where ΔHf, p and ΔHf, R are the enthalpies of formation for products and reactants at 298 K.
| $ {\Delta _{\rm{f}}}H_{298}^\Theta = \Delta {E_{298}} + \Delta (\mathit{pV}) = \Delta {E_0} + \Delta ZPE + \Delta {H_{\rm{T}}} + \Delta nRT $ | (2) |
Here ΔE0 stands for the change in total energy between products and reactants; ΔZPE is the difference between the zero-point energies of products and reactants at 0 K; ΔHT is the thermal corrections from 0 to 298 K. For the isodesmic reaction, Δn is 0 and Δ(pV) equals zero.
The accurately-determinated enthalpy of formation data from 1, 3, 5-trinitrobenzene, 1, 1′-azobis(2, 4, 6-trinitrobenzene), pyrrole, pyrazole, imidazole, 1, 2, 3-triazole, 1, 2, 4-triazole, and 1H-tetrazole were used to construct isodesmic reaction for 15 compounds (see Scheme 2).
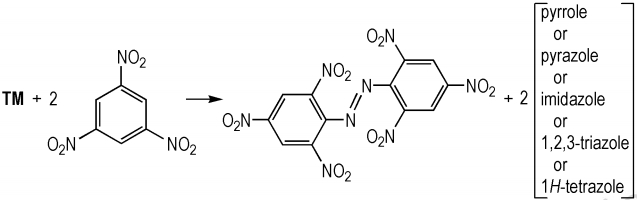
|
Scheme2 Isodesmic reaction for calculation of the standard enthalpy of formation |
As the compounds have similar structures excluding the azole rings, our study was focused on them.
The bond lengths, bond angles, and dihedral angles of azoles obtained from the calculation were listed in Table 1. It could be seen from Table 1 that most bonds (C—C, C—N, and N—N) in the azoles had close bond lengths (1.30-1.40 Å), which was between the length of typical single bond (1.46 Å) and typical double bond (1.22 Å). At the same time, the dihedral angles data indicated that all of the azoles were planar. It could be concluded that both the nitrogen and carbon atoms in the azoles were sp2 hybrid and the azole rings acquired planar aromatic structure as expected.
| Tab.1 Bond lengths (Å), bond angles (°), and dihedral angles (°) of azoles in the 15 azo-bridged azoles calculated at B3LYP/6-311+G (d, p) level |
It could be seen that the azo group also had effect on the structure of azoles, especially the atom connected to it. All the bonds consisted of and opposite this atom were elongated. And if the atom is nitrogen, the effect would be enlarged on bonds consisted of same kind atoms (i.e. C—C or N—N bond).
3.2 Enthalpies of FormationThe enthalpies of formation of the 15 compounds were investigated using the isodesmic reaction, which shown in Scheme 2, and the results were listed in Table 2. Enthalpies of formation of reference species acquired from same reactions were shown in Table 3.
| Tab.2 Enthalpies of reaction calculated by the B3LYP/6-311+G(d, p) theoretical method, and enthalpies of formation calculated using the isodesmic reaction |
| Tab.3 Enthalpies of formation for the reference species in the isodesmic reactions |
It could be observed that the enthalpies of formation of the azo-bridged azoles steadily increased with the increase of nitrogen contents, although some with higher nitrogen contents only had slight higher enthalpy of formation than the lower nitrogen content one.
As to the azoles with same number of nitrogen atoms, the enthalpies of formation decreased as the distance from azo group to the nitrogen atoms in azole rings increased. The increase of the distance between nitrogen atoms in azole rings had similar effect. Moreover, the effect from the latter was more prominent than the former. However, TM3 was an exception. Its enthalpy of formation was higher than TM2, and the degree of the conjugation might act as an important role (see Fig. 1 and Table 1). TM2 had greater conjugation, and then better stability, so its enthalpy of formation was lower than TM3.
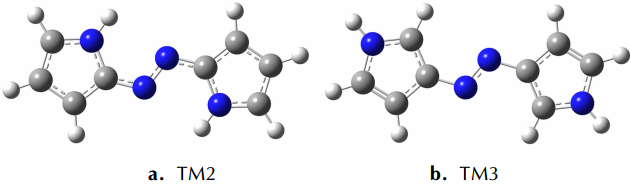
|
Fig.1 Optimized structures of TM2 and TM3 by B3LYP/6-311+G(d, p) theoretical method |
If the 15 azo-bridged azoles were arranged in an order of the nitrogen atoms they contented and then the position connected to the azo group (from 1 to 5), a scatter diagram of enthalpies of formation against the series could be obtained (see Fig. 2). It could be observed from the diagram that enthalpies of formation of TM1, TM4, TM10, and TM14 were higher than those of the compounds with same nitrogen content, and there was a good linear relationship. Of the four compounds, all of their nitrogen atoms connected each other to form a continuous nitrogen chain consisted of 4, 6, 8 and 10 nitrogen atoms respectively. So according to above principles, we could guess that 1, 1′-azobis (pentazole) might have the largest enthalpy of formation among the azo-bridged azoles.
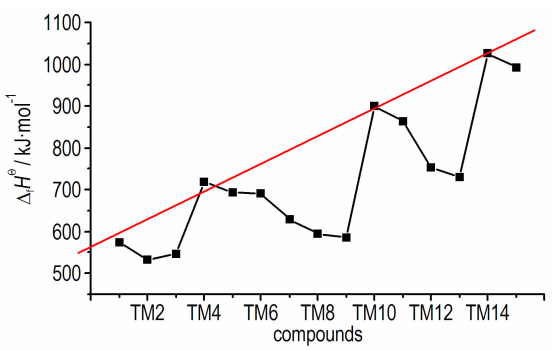
|
Fig.2 Enthalpies of formation of the azo-brideged azoles |
Entropies (SΘ) and constant pressure specific heat capacity (cp) for T=300-1500 K of 15 compounds were also calculated using B3LYP/6-311+G(d, p) theoretical method, and the results were shown in Table 4. It could be concluded that there was no correlation between the entropies with the nitrogen content, and all of the compounds had close values of 395.36-411.39 J·mol-1·K-1.
| Tab.4 Symmetry point group, entropy and specific heat capacity for the 15 compounds from B3LYP/6-311+G(d, p) calculations |
Under the calculated temperature, the constant pressure specific heat capacity of the azo-bridged azoles with same nitrogen content were nearly a constant. So four azo-bridged azoles (TM1, TM4, TM10, and TM14) were chosen to represent the ones contented 4, 6, 8 and 10 nitrogen atoms respectively for studying the relationship between the constant pressure specific heat capacity, nitrogen content and temperature. From the obtained scatter diagram (Fig. 3), it could be seen that the specific heat capacity decreased while the nitrogen content increased, and the tendency was obvious as temperature rose. According to this principle, it could be drawn that 1, 1′-azobis (pentazole) might have the lowest value of specific heat capacity among the azo-bridged azoles.
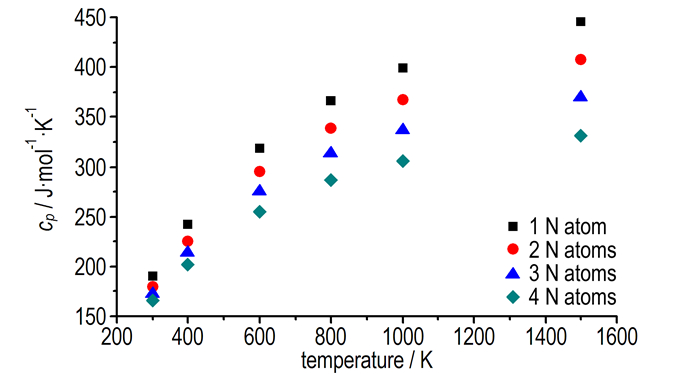
|
Fig.3 The cp of four typical compounds with 1 to 4 nitrogen atoms in the heterocycle as a function of temperature |
(1) The optimized structures and thermochemical properties of 15 azo-bridged azoles were theoretically obtained via a density functional theory method.
(2) All the nitrogen and carbon atoms of azoles were sp2 hybrid, and all of the heterocycles were planar aromatic rings.
(3) The enthalpies of formation increased uniformly with the numbers of nitrogen atoms.
(4) For the compounds with same number of nitrogen atoms, the enthalpies of formation decreased uniformly with increasing the distance from azo group to the nitrogen atoms in rings and the distance between the nitrogen atoms in rings.
(5) The constant pressure specific heat capacity at different temperatures are inversely proportional to the nitrogen content (the number of nitrogen atom on the heterocyclic ring).
| [1] |
Huynh M H V, Hiskey M A, Chavez D E, et al. Synthesis characterization and energetic properties of diazido heteroaromatic high-nitrogen C—N compound[J]. J Am Chem Soc, 2005, 127(36): 12537-12543. DOI:10.1021/ja0509735 |
| [2] |
Abe T, Tao G H, Joo Y H, et al. Activation of the C=F bond: transformation of CF3N=N-into 5-azidotetrazoles[J]. Angew Chem, 2008, 120(37): 7195-7198. DOI:10.1002/ange.v120:37 |
| [3] |
Klapötke T M, Mayer P, Schulz A, et al. 1, 5-Diamino-4-methyltetrazolium dinitramide[J]. J Am Chem Soc, 2005, 127(7): 2032-2033. DOI:10.1021/ja042596m |
| [4] |
He C L, Shreeve J M. Energetic materials with promising properties: synthesis and characterization of 4, 4′-bis(5-nitro-1, 2, 3-2H-triazole) derivatives[J]. Angew Chem Int Ed, 2015, 54(21): 6260-6264. DOI:10.1002/anie.201412303 |
| [5] |
Chavez D E, Hiskey M A, Gilardi R D. 3, 3′-Azobis(6-amino-1, 2, 4, 5-tetrazine): a novel high-nitrogen energetic material[J]. Angew Chem, 2000, 112(10): 1861-1863. DOI:10.1002/(ISSN)1521-3757 |
| [6] |
Huynh M H V, Hiskey M A, Hartline E L, et al. Polyazido high-nitrogen compounds: hydrazo-and azo-1, 3, 5-triazine[J]. Angew Chem, 2004, 116(37): 5032-5036. DOI:10.1002/(ISSN)1521-3757 |
| [7] |
Hammerl A, Klapötke T M, Nöth H, et al. [N(2)H5]2+[N(4)C—N=N—CN(4)]2-: A new high-nitrogen high-energetic material[J]. Inorg Chem, 2001, 40(14): 3570-3575. DOI:10.1021/ic010063y |
| [8] |
Martin B K, Norbert E, Helmut S, et al. Gas generating mixture containing copper diammine dinitrate: US5663524[P], 1997.
|
| [9] |
Hammerl A, Hiskey M A, Holl G, et al. Azidoformamidinium and guanidinium 5, 5′-azotetrazolate salts[J]. Chem Mater, 2005, 17(14): 3784-3793. DOI:10.1021/cm050684f |
| [10] |
Li Y C, Qi C, Li S H, et al. 1, 1′-Azobis-1, 2, 3-triazole: a high-nitrogen compound with stable N8 structure and photochromism[J]. J Am Chem Soc, 2010, 132(35): 12172-12173. DOI:10.1021/ja103525v |
| [11] |
Klapötke T M, Piercey D G. 1, 1′-Azobis(tetrazole): a highly energetic nitrogen-rich compound with a N10 Chain[J]. Inorg Chem, 2011, 50(7): 2732-2734. DOI:10.1021/ic200071q |
| [12] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09[CP], Gaussian Inc, Wallingford CT, 2009.
|
| [13] |
Scott D W, Berg W T, Hossenlopp I A, et al. Pyrrole: chemical thermodynamic properties[J]. J Phys Chem, 1967, 71(7): 2263-2270. DOI:10.1021/j100866a046 |
| [14] |
Jimenez P, Roux M V, Turrion C. Thermochemical properties of N-heterocyclic compounds I enthalpies of combustion vapour pressures and enthalpies of sublimation and enthalpies of formation of pyrazole imidazole indazole and benzimidazole[J]. J Chem Thermodyn, 1987, 19(9): 985-992. DOI:10.1016/0021-9614(87)90045-0 |
| [15] |
Bedford A F, Edmondson P B, Mortimer C T. Heats of formation and bond energies part Ⅵ n-butylisobutyraldimine n-butylisobutylamine pyrazole and imidazole[J]. J Chem Soc, 1962, 87: 2927-2931. |
| [16] |
Gabriel S, Eric E M, Joseph W B. Quantum chemical study of the structure and thermochemistry of the five-membered nitrogen-containing heterocycles and their anions and radicals[J]. J Phys Chem A, 2006, 110(51): 13979-13988. DOI:10.1021/jp065150w |
| [17] |
Kabo G J, Kozyro A A, Krasulin A P, et al. Thermodynamic properties and tautomerism of tetrazole[J]. J Chem Thermodyn, 1993, 25(4): 485-493. DOI:10.1006/jcht.1993.1156 |
| [18] |
Stull D R, Westrum E F, Sinke G C. The chemical thermodynamics of organic compounds[M]. New York: John Wiley & Sons Inc, 1969, 807-810.
|
| [19] |
Bathelt H, Volk F, Weindel M. The ICT-database of thermochemical values 4th ed[M]. 1997.
|

The structures and thermochemical properties of 15 kinds of azo-bridged azoles were studied by a density functional theory method to uncover the relationship between the structures and properties. B3LYP/6-311+G(d, p) level of theory was used to optimize the structures and calculate the thermochemical properties. The effect of the number and position of nitrogen atoms in azoles was discussed.