Lead azide and lead styphnate are the most commonly used primary explosives today. The long-term use of these compounds has caused considerable lead contamination for environment, which implies that it is necessary to look for greener primary explosives[1]. Nitrogen-rich heterocyclic compounds are new generation primary explosives[2]. Owing to the high positive heats of formation resulting from the large number of N—N and C—N bonds and the high level of environmental compatibility, 1, 2, 4-triazole energetic compounds have been studied over the last couple of years with growing interest[3-5]. Herein, a novel energetic inner-salt 5, 5′-bis(3-diazo-1, 2, 4-triazole)(BDTZ) was firstly synthesized, and its structure was well characterized by the means of NMR, IR and elemental analysis. Moreover, the performances of BDTZ such as detonation pressure and detonation velocity were calculated by Kamlet-Jacobs formulae[6]. BDTZ, which does not contain toxic heavy metals, exhibits good performances and potentially useful prospect as green primary explosive.
2 Experimental 2.1 Materials and InstrumentsThe oxalic acid and aminoguanidinium bicarbonate were of AR grade and purchased from the trade without further purification. 1H NMR and 13C NMR were obtained in DMSO-d6 on a Bruker AV500 NMR spectrometer. Infrared spectra were obtained from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Elemental analyses (C, H and N) were performed on a VARI-El-3 elementary analysis instrument.
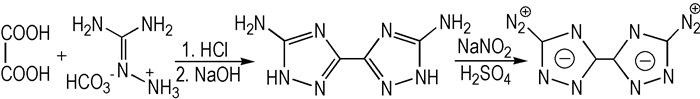
2.2 Synthetic RouteUsing oxalic acid and aminoguanidinium bicarbonate as starting materials, the title compound BDTZ was firstly synthesized via the reactions of cyclodehydration and diazotization (Scheme 1).
|
Scheme1 The synthetic route of 5, 5′-bis(3-diazo-1, 2, 4-triazole) |
According to a modified literature procedure[5], hydrochloric acid (25 mL) was added to a stirred mixture of oxalic acid (10.0 g, 79.4 mmol), aminoguanidinium bicarbonate (22.6 g, 166 mmol) and water (25 mL). The reaction mixture was stirred at 70 ℃ for 1 h. After cooling to 40 ℃, the mixture was made alkaline to pH=14 with sodium hydroxide. The reaction mixture was heated at reflux for another 1 h and subsequently acidified with acetic acid to pH=4. The resulting precipitate was collected by filtration, washed with water and dried in vacuum to yield white solid 9.9 g with a yield of 75.1%.
1H NMR(DMSO-d6, 500 MHz): 5.92(s, 4H, NH2), 12.77(s, br, 2H, NH); IR(KBr, ν/cm-1): 3407, 3335, 3094, 2862, 2782, 1709, 1663, 1600, 1485, 1457, 1359, 1268, 1105, 1061, 987, 798, 733; Anal.Calcd. for C4H6N8(%): C 28.92, H 3.64, N 67.44; Found: C 29.16, H 3.70, N 67.14.
2.4 Synthesis of 5, 5′-Bis(3-diazo-1, 2, 4-triazole)(BDTZ)A suspension of 5, 5′-diamino-3, 3′-bis-1, 2, 4-triazole (0.50 g, 3.01 mmol)in 20% sulfuric acid (15 mL) was added dropwise to a solution of sodium nitrite (3 equiv., 0.63 g, 9.13 mmol) in water (5 mL) at 0-5 ℃. Then the reaction mixture was stirred at room temperature for 1 h. The resulting white precipitate was filtered, washed with water, and dried in air to yield white solid 0.4 g with a yield of 70.6%.
13C NMR (125 MHz, DMSO-d6, δ): 156.411, 136.762; IR(KBr, ν/cm-1): 3441, 2277, 2232, 1721, 1402, 1326, 1311, 1275, 1205, 1074, 1016, 970, 706, 655. Anal.Calcd. for C4N10(%): C 25.53, N 74.47; Found: C 25.42, N 73.29.
2.5 The Performances of Physico-chymistry and Detonation for BDTZThe density of BDTZ was computed based on Monte-Caolo method[7-8] using the optimized structure at the B3LYP/6-311G+(d, p) level of theory. The enthalpies of the gas phase species M were computed according to the atomization energy method[9] by using the CBS-4M method. Gas phase enthalpies were transformed to solid state enthalpies by Trouton′s rule[10]. Based on the calculated enthalpy of formation and density, the detonation velocity and detonation pressure for BDTZ were calculated by Kamlet-Jacobs formulae[6]. The performances for BDTZ were showed in Table 1.
| Tab.1 Comparison of the performances for lead azide and BDTZ |
All tests point to the fact that this material is a suitable and non-toxic replacement for lead azide, with a straightforward synthesis from commonly available chemicals. Above all, a novel energetic inner-salt, 5, 5′-bis(3-diazo-1, 2, 4-triazole)(BDTZ), was designed and synthesized via a two-step reaction process, and its structure was fully characterized for the first time. In addition, some main performances of energetic inner-salt BDTZ were obtained by calculation as follows: Its density is 1.73 g·cm-3, detonation velocity is 7780 m·s-1 and enthalpy of formation is 1256.7 kJ·mol-1.
| [1] |
Dennis Fischer, Thomas M, Klapötke, Jorg Stierstorfer. Potassium 1, 1′-dinitramino-5, 5′-bistetrazolate: a primary explosive with fast detonation and high initiation power[J]. Angew Chem Int Ed, 2014, 53: 8172-8175. DOI:10.1002/anie.201404790 |
| [2] |
SHENG Di-lun, ZHU Ya-hong, PU Yan-li. Development of a new-generation primary explosives designing and synthesis[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(3): 263-272. |
| [3] |
Alexander A. Dippold, Thomas M., Klapötke, Nils Winter. Insensitive nitrogen-rich energetic compounds based on the 5, 5-dinitro-3, 3-bi-1, 2, 4-triazol-2-ide anion[J]. Eur J Inorg Chem, 2012, 3474-3484. |
| [4] |
Alexander A Dippold, Thomas M, Klapötke. A study of dinitro-bis-1, 2, 4-triazole-1, 1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N—oxides[J]. J Am Chem Soc, 2013, 135: 9931-9938. DOI:10.1021/ja404164j |
| [5] |
LUO Yi-fen, WANG Zi-jun, BI Fu-qiang, et al. Synthesis and properties of insensitive energetic material MAD-X1[J]. Chinese Journal of Explosives & Propellants, 2015, 38(5): 13-17. |
| [6] |
Kamlet M J, Jacobs S J. Chemistry of Detonations I. A simple method for calculating detonation properties of CHNO explosives[J]. J Chem Phys, 1968, 48(1): 23-35. DOI:10.1063/1.1667908 |
| [7] |
Qiu L, Xiao H M, Gong X D, et al. Crystal density predictions for nitramines based on quantum chemistry[J]. Journal of Hazardous Materials, 2007, 141(1): 280-288. DOI:10.1016/j.jhazmat.2006.06.135 |
| [8] |
Rice B M, Hare J J, Byrd E F E. Accurate predictions of crystal densities using quantum mechanical molecular volumes[J]. J Chem Phys A, 2007, 111(42): 10874-10879. DOI:10.1021/jp073117j |
| [9] |
Montgomery J.J. A., Frisch M.J., Ochterski J.W., et al. A complete basis set model chemistry. Ⅶ. use of the minimum population localization method[J]. J Chem Phys, 2000, 112: 6532-6542. DOI:10.1063/1.481224 |
| [10] |
Westwell M S, Searle M S, Wales D J, et al, Empirical correlations between thermodynamic properties and intermolecular forces[J]. J Am Chem Soc, 1995, 117: 5013-5015.
|

A novel energetic inner-salt 5, 5′-bis(3-diazo-1, 2, 4-triazole) was firstly designed and synthesized by cyclodehydration, diazotization reactions with a total yield of 53.0%, and its structure was confirmed. Theoretical calculations at 3LYP/6-311G+(d, p) level were performed, and the results show that the density is 1.73 g·cm-3, the Dv is 7780 m·s-1 and Dp is 26.72 GPa.




