3, 4-Bis(3-nitrofurazan-4-yl)furoxan (DNTF, Fig. 1) is a novel energetic material with high density, high energy and simple synthesis technology. Further study shows that its detonation performance is more superior than octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine (HMX) but close to 1, 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (CL-20)[1]. Since it was synthesized, researchers mainly focused on its synthesis[2], crystal structure[3] and comprehensive performance[4-6].

|
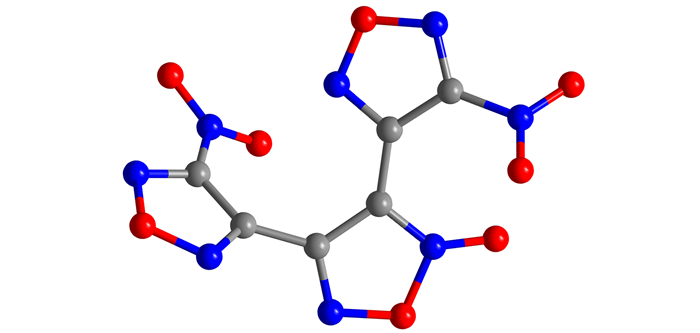
Fig.1 Chemical structure of DNTF |
Solution crystallization process is an important and traditional way to enhance the purity and morphology of solid products. What′s more, the solubility is an extremely significant thermodynamic data for crystallization and calculation other thermodynamic parameters. Besides, solubility can affect the capacity of the crystallization process, as well as its ability to reject undesired compounds and minimize loss in the mother liquor. Ethanol is the most widely used organic solvent because of inexpensive, non-toxic and environmental-friendly. Moreover, the solubility of DNTF is moderate and obviously changes with temperature and concentration in ethanol. Therefore, ethanol can be used as the solvent for crystallizing DNTF and it′s necessary to study the solubility of DNTF in binary system ethanol-water.
In this work, the solubility of DNTF in six different concentration ethanol solvents at 298.15-338.15 K is measured. Four common correlation equations are adopted to correlate the experimental values. The standard enthalpy of dissolution, standard entropy of dissolution and standard Gibbs free energy are calculated according to the experimental data.
2 Experimental 2.1 Chemical MaterialsDNTF sample was provided by Xi′an Modern Chemistry Research Institute. It was purified by recrystallization in alcohol and its mass fraction purity, measured by High Performance Liquid Chromatography, was greater than 0.995. Ethanol of analytical reagent grade was purchased from local reagent factory without further purification whose mass fraction purity was no less than 0.995. Deionized water was made by a Millipore Mili-Q Plus water system.
2.2 Single Crystal Structure DeterminationThe transparent single crystal of DNTF was cultivated from binary system ethanol-water and was characterized by using the single crystal X-ray diffraction analysis, the molecular structure was shown in Fig. 2. The results show that the crystal is orthorhombic with and the crystal parameters are same with reference [3].
|
Fig.2 The molecular structure of DNTF |
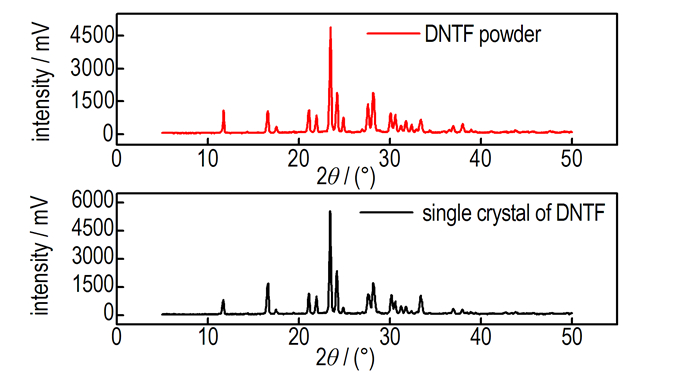
Since the solubility may change with the crystal structure, it′s necessary to confirm the crystal structure before the measurement of its solubility. The DNTF single crystal and DNTF powder used to measure the solubility were identified by powder X-ray diffraction (PXRD) which was carried out by using CuKα radiation (1.54 Å) within 2θ range of 5° to 50° on Rigaku D/max-2500 (Rigaku, Japan) at a scanning rate of 1 step·s-1. The result of PXRD was shown in Fig. 3 which illustrate clearly that the crystallinity and the crystal form of the powder DNTF used to measure the solubility are the same with the single crystal cultivated from binary system ethanol-water.
|
Fig.3 Powder X-ray diffraction of DNTF |
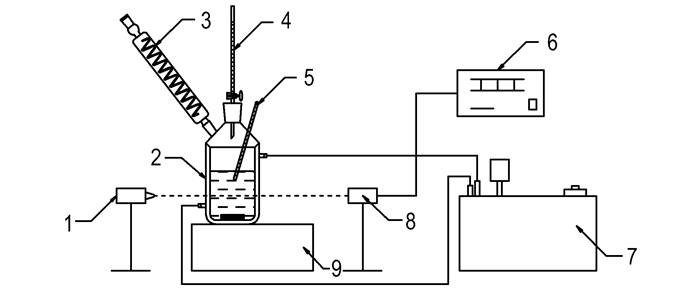
The laser monitoring system (Fig. 4), the same method with Shi et al[7], was used to detect the phase transformation point at a constant temperature. The procedure of solubility measurement is same with Lan et al[8]. The solubility of DNTF in binary system ethanol-water within 298.15-338.15 K expressed by mole fraction was calculated by equation (1), the initial mole fraction composition of the binary solvent mixtures was defined by equation (2):
| $ {x_1} = \frac{{{m_1}/{M_1}}}{{{m_1}/{M_1} + {m_2}/{M_2} + {m_3}/{M_3}}} $ | (1) |
| $ {x_2} = \frac{{{m_2}/{M_2}}}{{{m_2}/{M_2} + {m_3}/{M_3}}} $ | (2) |
|
Fig.4 Flow diagrams of experiment 1—laser generator, 2—glass vessel, 3—condenser pipe, 4—burette, 5—mercury thermometer, 6—digital display, 7—thermostatic bath, 8—photoelectric switch, 9—magnetic stirrer |
where x1 is the solubility (solid-liquid equilibria) of DNTF in mole fraction; x2 is the solute-free mole fraction of ethanol in the binary liquid solvents, m1, m2, m3 are the mass of DNTF, ethanol, water, respectively, M1, M2, M3 are the molecular mass of DNTF, ethanol, water, respectively.
3 Results and discussion 3.1 Measured SolubilitySolubility values of DNTF measured in different concentrationbinary system ethanol-water at temperatures ranging from 298.15 K to 338.15 K are listed in Table 1.
| Tab.1 Experimental mole fraction solubility values x1i and calculated solubility values xci of DNTF in binary system ethanol-water at temperature T and pressure p=0.1 MPa1) |
Since solid-liquid equilibrium is usually not available, correlation and prediction schemes are frequently utilized.The van′t Hoff plot, modified Apelblat equation, R-K model and Jouyban-Acree model are adopted to correlate the experimental values. Root-mean-square deviation (RMSD) is used to evaluate the fitting results of the correlation equation. The RMSD is defined as:
| $ {\rm{RMSD}} = {\left[{\frac{1}{N}\sum\limits_{i = 1}^N {{{({x_{{\rm{c}}i}}-{x_{1i}})}^2}{\rm{ }}} } \right]^{1/2}} $ | (3) |
where N represents the total number of experimental points, x1i is the experimental data, xci is the calculated values.
3.2.1 van′t Hoff plotAssuming the solution is an ideal solution (γ = 1), then the solubility of DNTF can be correlated by the van′t Hoff plot[9-10] which reflects the relationship between the mole fraction of a solute and the temperature when the solvent effect is considered. The van′t Hoff plot can be described as follows:
| $ {\rm{ln}}{x_1} = A + B/T $ | (4) |
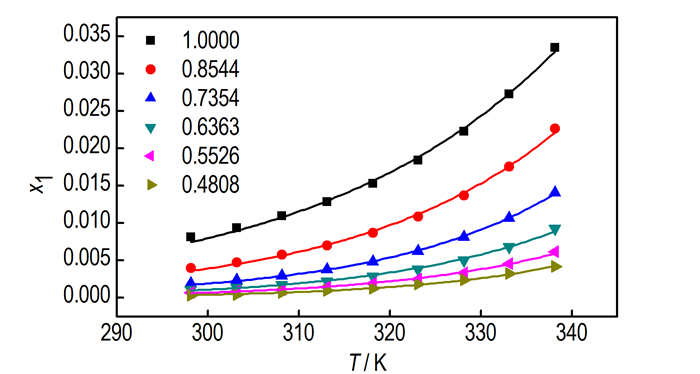
whereT is the absolute temperature of experiment, A and B are the model parameters. Table 2 lists the model parameters values of A, B, the correlation coefficient of fitting results and RMSD. Fig. 5 is the fitting curves correlated by equation (4), which graphically illustrates the function between lnx1 and 1/T. From Fig. 5 we can clearly see that it is a linear relationship between lnx1 and 1/T, the RMSD of the correlation results are acceptable. So the ideal equation can be used to correlate the solubility of DNTF in binary system ethanol-water well.
| Tab.2 Model parameters, correlation coefficient and RMSD of van′t Hoff plot |

|
Fig.5 Solubility of DNTF in ethanol-water binary system correlated with different temperatures.The points represent the experimental data. Curves are calculated according to Eq.(4) using the van′t Hoff plot |
The modified Apelblat equation[11-13] deduced from the Clausius-Clapeyron equation is a semiempirical equation, which has been widely used in correlation the experimental solubility values. The equation can be expressed as:
| $ {\rm{ln}}{x_1} = A + B/T + C{\rm{ln}}T $ | (5) |
where A, B and C are the model parameters. Table 3 lists the model parameter values of A, B, C, R2 and the RMSD. The experimental solubility values of DNTF in ethanol-water binary system at different temperatures and the solubility curve fitted by the modified Apelblat equation are shown in Fig. 6. It is clear that modified Apelblat equation demonstrates good consistency with the experimental values at different temperatures. Besides, the correlation coefficient of each concentration ethanol is close to 1 and the RMSD is micro, so the modified Apelblat equation can correlate the solubility of DNTF in binary system ethanol-water at different temperatures precisely.
| Tab.3 Model parameters, R2 and RMSD of modified Apelblat equation |
|
Fig.6 Solubility of DNTF in binary system ethanol-water correlated with different temperatures.The points represent the experimental data. Curves are calculated according to Eq.(5) using the Modified Apelblat equation |
The R-K model[14-15] is one of the best models used to calculate the solute solubility in binary solvents and it is expressed as follows:
| $ {\rm{ln}}{x_1} = {x_2}{\rm{ln}}{\left( {{x_1}} \right)_2} + {x_3}{\rm{ln}}{\left( {{x_1}} \right)_3} + {x_2}{x_3}\sum\limits_{i = 1}^N {{S_i}\left( {{x_2}-{x_3}} \right)} $ | (6) |
where x1 is the solubility of DNTF, x2 and x3 stand for the initial molar fraction composition of ethanol and water in binary solvent mixtures when the solute is not added, (x1)2 and (x1)3 are the mole fraction solubility of DNTF in pure ethanol and water, respectively. Si is the model constant and N can be 0, 1, 2 or 3[16]. For binary solvents system, substituting N=2 and x3=1-x2 into Eq.(6) can give a simplified equation as follows.
| $ {\rm{ln}}{x_1} = {B_0} + {B_1}{x_2} + {B_2}x_2^2 + {B_3}x_2^3 + {B_4}x_2^4 $ | (7) |
where B0, B1, B2, B3, and B4 are the model parameters. The model parameters values of B0, B1, B2, B3, B4, R2 and the RMSD are displayed in Table 4. The solubility data calculated by the R-K equation are graphically displayed in Fig. 7 which illustrates clearly to us that the solubility values correlated by the R-K equation are in good agreement with the experimental ones. Besides, the R2 and RMSD are acceptable, so the R-K equation can be used to correlate the solubility of DNTF in ethanol-water binary system at different concentration accurately.
| Tab.4 Model parameters, R2 and RMSD of R-K equation |
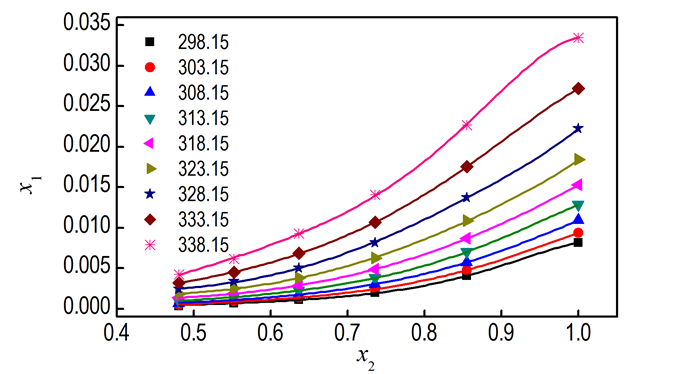
|
Fig.7 Mole fraction solubility of DNTF in binary system ethanol-water at various temperatures. The points represent the experimental data. Curves are calculated according to Eq.(7) using the R-K equation |
The Jouyban-Acree model[17-18] is also widely used to describe the solubility of DNTF in the whole temperature range by taking both composition of solution and temperature into consideration. The model is expressed as follows:
| $ {\rm{ln}}{x_1} = {x_2}{\rm{ln}}{\left( {{x_1}} \right)_2} + {x_3}{\rm{ln}}{\left( {{x_1}} \right)_3} + {x_2}{x_3}\sum\limits_{i = 1}^N {{J_i}\left( {{x_2}-{x_3}} \right)} /T $ | (8) |
where Ji is the model constant. Substitution of N=2 into Eq.(8) can give a new simplified equation as:
| $ \begin{array}{l} {\rm{ln}}{x_1} = {\rm{ }}{A_0} + {A_1}/T + {A_2}{\rm{ln}}T + {A_3}{x_2} + {A_4}{x_2}/T + {A_5}{\left( {{x_2}} \right)^2}/T + \\ {A_6}{\left( {{x_2}} \right)^3}/T + {A_7}{\left( {{x_2}} \right)^4}/T + {A_8}{x_2}{\rm{ln}}T \end{array} $ | (9) |
where A0-A8 are empirical model parameters, which can be obtained by least-squares analysis. The model parameters values of A0-A8, R2 and the RMSD are displayed in Table 5. The three three-dimensional diagram between x1and x2, T is shown in Fig. 8 which demonstrates to us that all of the experimental values are on the surface fitted by the Jouyban-Acree model, besides, the R2 is very close to 1 and the RMSD is close to 0. Therefore, the Jouyban-Acree model is a perfect equation to correlate the experimental solubility data of DNTF in ethanol-water binary system. What′s more, we can calculate the solubility of DNTF in ethanol-water binary system at random temperature and concentration by the Jouyban-Acree model obtained from this study.
| Tab.5 Parameters of the Jouyban-Acree model for the solubility of DNTF in binary solvent system of ethanol-water binary system |
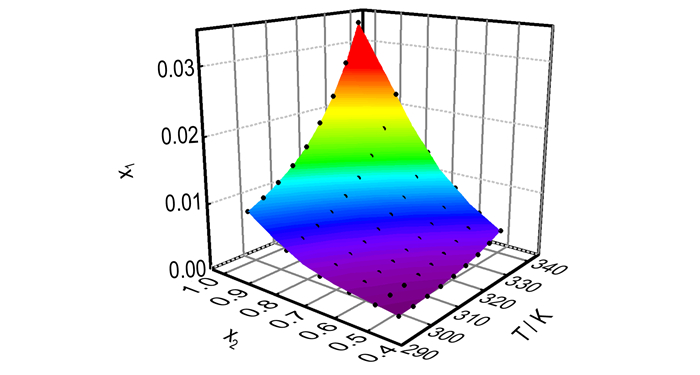
|
Fig.8 Experimental mole fraction solubility of DNTF in binary system ethanol-water. The points represent the experimental data, and the surface represents the results fitted by the Jouyban-Acree model |
Some thermodynamic properties such asthe standard enthalpy of dissolution, standard entropy of dissolution and the standard Gibbs free energy can be calculated by the solubility data measured. The function between the mole fraction solubility of DNTF and the absolute temperature can be expressed as[19]:
| $ {\rm{ln}}{x_1} =-\frac{{{\Delta _{{\rm{dis}}}}{H^\Theta }}}{{RT}} + \frac{{{\Delta _{{\rm{dis}}}}{S^\Theta }}}{{R}} $ | (10) |
where R represents the gas constant (8.3145 J·K-1·mol-1), ΔdisHΘand ΔdisSΘ are the standard enthalpy of dissolution and standard entropy of dissolution of DNTF. Assuming the lnx1 is dependent variable, 1/T is independent variable, so the lnx1 is linear relation with 1/T and we can calculate the ΔdisHΘ from the slope and ΔdisSΘ from the intercept of the linear equation displayed in Fig. 5. The standard Gibbs free energy of dissolution of DNTF in different solvents can be calculated as:
| $ {\Delta _{{\rm{dis}}}}{G^\Theta } = {\Delta _{{\rm{dis}}}}{H^\Theta }-\mathit{T}{\Delta _{{\rm{dis}}}}{S^\Theta } $ | (11) |
In this work, we calculated change in Gibbsfree energy at 318.15 K as a mean temperature (ΔdisGmeanΘ).
The relative contributions from enthalpy %ξH and entropy %ξS to the standard free Gibbs energy of the solution are define by the following two equations[20]:
| $ \% {\xi _H} = \frac{{\left| {{\Delta _{{\rm{dis}}}}{H^\Theta }} \right|}}{{\left| {{\Delta _{{\rm{dis}}}}{H^\Theta }} \right| + \left| {T{\Delta _{{\rm{dis}}}}{S^\Theta }} \right|}} \times 100 $ | (12) |
| $ \% {\xi _S} = \frac{{T{\Delta _{{\rm{dis}}}}{S^\Theta }}}{{{\Delta _{{\rm{dis}}}}{H^\Theta } + T{\Delta _{{\rm{dis}}}}{S^\Theta }}} \times 100 $ | (13) |
The calculated enthalpy, entropy, and Gibbs energy of dissolution together with %ξH, %ξS calculated under the mean temperature are displayed in Table 6. Since the values of ΔdisHΘ are positive in all solvents, the dissolution of DNTF in binary system ethanol-water is an endothermic process. In addition, the dissolution of DNTF is a non-spontaneous process because the values of ΔdisGmeanΘ are positive. Moreover, the main contributor to the standard molar Gibbs energy of dissolution is the enthalpy instead of entropy in that the %ξH is greater than %ξS.
| Tab.6 Standard enthalpy, entropy of the dissolution of DNTF in different solvents and the standard Gibbs free energy of solution at mean temperature (318.15 K) |
(1) The solubility values of DNTF increase with increasing the ratio of ethanol and temperature as a nonlinear function.
(2) Four correlation equations selected in this study all can be used to fit the solubility values of DNTF precisely. What′s more, all of the 54 experimental values are used to correlate the parameters of Jouyban-Acree model and the correlated results are better than other three equations by comparing the R2 and RMSD.
(3) Some important thermodynamic properties such as the standard enthalpy of dissolution, standard entropy of dissolution and standard Gibbs free energy of dissolution have been calculated.
| [1] |
ZHENG Wei, WANG Jiang-ning, Han Fang, et al. Chemical stability of CMDB propellants containing DNTF[J]. Chin J Explos Propell, 2010, 33: 10-13. |
| [2] |
Zhou Yanshui, Wang Bozhou, Li Jiangkang, et al. Study on synthesis, characterization and properties of 3, 4-Bis(4′-nitrofurazano-3′-yl)furoxan[J]. Acta Chim Sinica, 2011, 69: 1673-1680. |
| [3] |
ZHOU Yan-shui, ZHANG Zhi-zhong, LI Jian-kang, et al. Crystal structure of 3, 4-dinitrofurazanofuroxan[J]. Chin J Explos Propell, 2005, 28: 43-46. |
| [4] |
Sinditskii V P, Burzhava A V, Sheremetev A B, et al. Thermal and combustion properties of 3, 4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)[J]. Propell Explos Pyrot, 2012, 37: 575-580. DOI:10.1002/prep.v37.5 |
| [5] |
WANG Qin-hui. Properties of DNTF-based melt-cast explosives[J]. Chin J Explos Propell, 2003, 26: 57-59. |
| [6] |
Zhao Fengqi, Chen Pei, Hu Rongzu, et al. Thermochemical properties and non-isothermal decomposition reaction kinetics of 3, 4-dinitrofurazanfuroxan(DNTF)[J]. J Hazard Mater, 2004, 113: 67-71. DOI:10.1016/j.jhazmat.2004.07.009 |
| [7] |
Shi Xiaohua, Zhou Cairong, Gao Yuguo, et al. Measurement and correlation for solubility of (S)-(+)-2, 2-dimethyl-cyclopropane carbox amide in different solvents[J]. Chin J Chem Eng, 2006, 14: 547-550. DOI:10.1016/S1004-9541(06)60112-2 |
| [8] |
Lan Guanchao, Wang Jianglong, Chen Lizhen, et al. Measurement and correlation of the solubility of 3, 4-bis(3-nitrofurazan-4-yl)furoxan(DNTF) in different solvents[J]. J Chem Thermodyn, 2015, 89: 264-269. DOI:10.1016/j.jct.2015.06.003 |
| [9] |
Yu Chao, Zeng Zuoxiang, Xue Weilan. Measurement and correlation of the solubility of hexaquonickel(Ⅱ) bis(p-toluenesulfonate) in water + ethanol solvents within 288.15-333.15 K[J]. Ind Eng Chem Res, 2015, 54: 3961-3967. DOI:10.1021/ie5049753 |
| [10] |
Jiang Linkun, Wang Lisheng, Du Chaojun, et al. Measurement and correlation of the solubilities of tetra(5, 5-dimethyl-1, 3-dioxaphosphorinanyl-2-oxy) neopentane in different pure solvents[J]. Fluid Phase Equilib, 2014, 367: 117-124. DOI:10.1016/j.fluid.2014.01.043 |
| [11] |
Apelblat A, Manzurola E. Solubilities of manganese, cadmium, mercury and lead acetates in water from T=278.15 K toT=340.15 K[J]. J Chem Thermodyn, 2001, 33: 147-153. DOI:10.1006/jcht.2000.0735 |
| [12] |
Zhou Li, Zhang Peipei, Yang Guangde, et al. Solubility of chrysin in ethanol and water mixtures[J]. J Chem Eng Data, 2014, 59: 2215-2220. DOI:10.1021/je5001654 |
| [13] |
Zhao Yan, Wang Yongli. Measurement and correlation of solubility of tetracycline hydrochloride in six organic solvents[J]. J Chem Thermodyn, 2013, 57: 9-13. DOI:10.1016/j.jct.2012.08.007 |
| [14] |
Liu Cheng, Tang Guiwei, Ding Hui, et al. Determination of the solubility and thermodynamic properties of wedelolactone in a binary solvent of ethanol and water[J]. Fluid Phase Equilib, 2015, 385: 139-146. DOI:10.1016/j.fluid.2014.10.031 |
| [15] |
Yu Jing, Ma Tianle, Li An, et al. Solubility of disodium cytidine 5′-monophosphate in different binary mixtures from 288.15 K to 313.15 K[J]. Thermochim Acta, 2013, 565: 1-7. DOI:10.1016/j.tca.2013.04.018 |
| [16] |
Chen Li-Zhen, Zhang Tian-Bei, Li Man, et al. Solubility of 2, 2′, 4, 4′, 6, 6′-hexanitrostilbene in binary solvent of N, N-dimethylformamide and acetonitrile[J]. J Chem Thermodyn, 2016, 95: 99-104. DOI:10.1016/j.jct.2015.12.004 |
| [17] |
Jouyban A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures[J]. J Pharm Pharmaceut Sci, 2008, 11: 32-58. |
| [18] |
Wang Shui, Qin Liying, Zhou Zhimao, et al. Solubility and solution thermodynamics of betaine in different pure solvents and binary mixtures[J]. J Chem Eng Data, 2012, 57: 2128-2135. DOI:10.1021/je2011659 |
| [19] |
Wang Na, Fu Qiang, Yang Guangde. Determination of the solubility, dissolution enthalpy and entropy of icariin in water, ethanol, and methanol[J]. Fluid Phase Equilib, 2012, 324: 41-43. DOI:10.1016/j.fluid.2012.03.022 |
| [20] |
Hu Yonghong, Liu Xiang, Yang Wenge, et al. Measurement and correlation of the solubility of 4-methylbenzoic acid in (methanol + acetic acid) binary solvent mixtures[J]. J Mol Liq, 2014, 193: 213-219. DOI:10.1016/j.molliq.2013.12.044 |
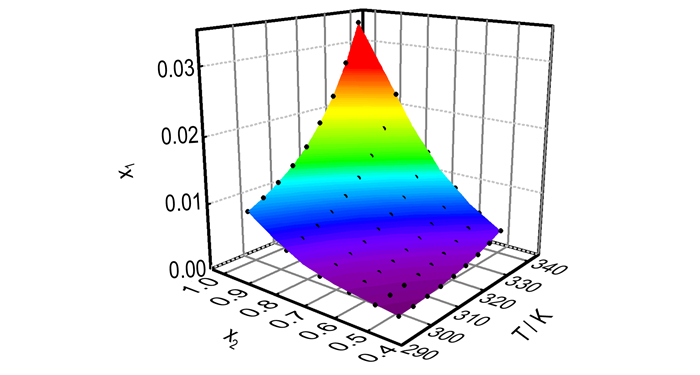
The solubility of DNTF from 298.15 K to 338.15 K was determined by using a laser monitoring system under atmospheric pressure. The relationship between the temperature and the ethanol ratio was obtained.




