2. Southwest University of Science and Technology, Mianyang 621010, China
2. 西南科技大学材料科学与工程学院, 四川 绵阳 621010
In the field of high explosives, many efforts have been focused on exploring more powerful, safer and environmentally friendly explosives. 1, 1-diamino-2, 2-dinitroethylene (C2H4N4O4), commonly referred to FOX-7, is a novel high explosive[1]. Compared with the most widely used and powerful explosive 1, 3, 5-trinitro-1, 2, 4-triazacyclohexane(RDX), FOX-7 combines two important aspects, i.e., high performance and low sensitivity[2]. However, FOX-7 possesses several polymorphs[3], which suppresses its application. It is well known that phase transitions of energetic materials commonly observed under high temperature directly affect the stability and performance[4]. Indeed, the most stable polymorph at ambient conditions is always sought owning to its highest detonation velocity[5]. Moreover, phase transitions induced by high temperature frequently result in crystal defects which can sensitize the explosive by forming hot spots and super-rate burning[5]. Therefore, developing a deeper understanding of phase behaviors can lead to valuable insight into the complex interplay of intra-and inter-molecular interactions which are responsible for shock-initiated chemical reactions.
Crystalline FOX-7 has three polymorphs, i.e. α, β and γ. The most stable phase at room temperature is α-FOX-7, and it can be converted to other polymorphs at higher temperatures, and upon cooling the explosive directly but incompletely restores to the α phase[6]. The phase transition behaviors of FOX-7 have been explored by X-ray diffraction methods, Raman, and thermal techniques[6-10]. However, the phase transition mechanism is still confusion. The effects of H-bonding and molecular structure might be the key for understanding the polymorphism. But the information on the behavior of the related H-bonding vibrations is not observed in FOX-7[11]. Indeed, the stability of FOX-7 mainly depends on the strong H-bonds between nitro oxygen atoms and amino hydrogen atoms. Furthermore, detailed analysis of the changes of H-bonding properties of the FOX-7 crystals under extreme conditions could provide important insight for understanding the detonation behavior of FOX-7 at molecular level[12]. Evers[7] and Crawford[8] have investigated the molecular structure and the effects of H-bonding of FOX-7 by X-ray single crystal methods. But few tools and techniques including X-ray single crystal diffraction could visually present the changes of H-bonding and molecular structure with increasing temperature in a single figure.
Recently, temperature-dependent Fourier transform infrared (FTIR) spectroscopy is recognized as a powerful technique for in-situ characterization of phase transitions of energetic material. Temperature-dependent FTIR spectroscopy could give information about intermolecular vibrational modes mediated by the H-bonding and access knowledge about the structure and vibrational dynamics of solids[13-15]. Pressure-induced changes of FOX-7 were observed by using FTIR spectroscopy[9], and the effects of H-bonding and molecular structure with pressures were discussed. Temperature-induced changes were investigated by Bishop[16] using IR. The H-bonding effects of FOX-7 with temperatures were investigated but the changes of molecular structure were ignored. Thus, we sought to conduct a FTIR study of FOX-7 to observe and compare the effects of H-bonding and molecular structure on molecular vibrations with temperatures and to investigate the phase transitions of FOX-7. This study would also offer the valuable insight into the interesting secondary explosive behaviors under high temperature conditions.
2 ExperimentalThe FOX-7 samples were provided by Institute of Chemical Materials, Chinese Academy of Engineering Physics. FTIR spectra were taken with KBr pellets in Model Nicolet 6700 spectrograph. The resolution was 1 cm-1, and the scan range was 400-4000 cm-1. Spectra were recorded during heating from 55 ℃ to 190 ℃ at a constant heating rate of 5 ℃·min-1. Then, the spectra were obtained during the cooling process.
Powder X-ray diffraction(XRD) patterns were recorded on a Bruker D8 Advance with a CuKα radiation (λ=1.5406 Å), the voltage and current applied are 40 kV and 40 mA, respectively. Samples of FOX-7 were heated from 55 ℃ to 190 ℃, and data were collected from 5° to 60° (2θ) with an increment of 0.02° and a continuing time of 0.1 s for each increment.
3 Results and Discussion 3.1 α→β Phase Transition 3.1.1 Bands Assignments for Vibrational ModesThe temperature-dependent FTIR spectra of FOX-7 heating from 55 ℃ to 122 ℃ in the region of 3500-3150, 1670-970 cm-1 and 900-400 cm-1 are displayed in Fig. 1. As shown in Fig. 1a, ν1, ν2 and ν3 have the tendency to shift toward higher wavenumber when the temperature increases. As shown in Fig. 1b, ν5 (1632 cm-1) shifts toward lower wavenumber with increasing temperature. Subsequently, ν6 overlaps or incorporates with ν5. The frequencies of bands appearing at 1526 (ν7), 1470 (ν8) and 1394 (ν9), 1350 (ν10) cm-1 remain nearly constant during heating (see in Fig. 1b). Generally speaking, the frequencies of FOX-7 in most vibrational bands show little changes during heating in the range of 55 ℃ to 105 ℃. The FOX-7 molecules show strong intermolecular H-bonds within the wave-shaped layers. When the H-bonding is reduced or grows weaker, the stretching vibrational band usually shifts to higher frequency, whereas the deformation vibrational band usually moves to lower frequency[17]. According to the frequency shifts of H-bonding interaction, vibrational modes are clearly assigned[18-24], listed in Table 1. Besides, ν4 can be assigned to Fermi resonance between the NH2 (νas) and the overtone mode of the NH2 scissor (2ν5), and the vibrational mode at~1620 cm-1 can be identified as a newly formed peak, which is assigned to Fermi resonance between NH2 and NO2 scissoring vibrations[25]. In contrast to other H-bonded energetic materials, such as 1, 3, 5-triamine-2, 4, 6-trinitrobenzene (TATB)[17], the weakening of H-bonding effect in most vibrational bands is inconspicuous with increasing temperature below 105 ℃.
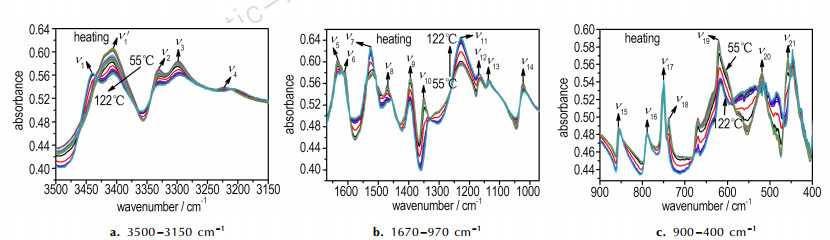
|
Fig.1 FTIR spectra of FOX-7 while the temperature increases from 55 ℃ to 122 ℃ |
| Tab.1 Assignments of vibrational bands in spectra of FOX-7 |
Above 105 ℃, the NH2 (νas) peak near 3443 cm-1 abruptly shifts to higher wavenumber, as shown in Fig. 1a. The intensity of NO2 (νs) ν10 sharply decreases and shifts toward lower wavenumber (Fig. 1b). As shown in Fig. 1c, ν18 disappears above 105 ℃. Spectral bands in the lower spectral range (650-467 cm-1) undergo significant changes in the range of 100 ℃ to 120 ℃. The smoothing ν20 is replaced by a broad medium strong peak above 105 ℃. The band at 458 cm-1 vanishes, and a peak at 447 cm-1 grows at about 111 ℃ and reaches the same intensity of the band near 458 cm-1. FTIR spectra of FOX-7 experience some abrupt changes on heating at 105 ℃, indicating that the α→β transition occurs.
The molecular conformations and packing arrangements of α and β polymorphs can provide some insight. The α and β-FOX-7 have similar structures of two-dimensional wave-shaped layers (CCDC, SEDTUQ03 and SEDTUQ06), as shown in Fig. 2. However, heating of the FOX-7 molecules to 120 ℃ flattens the wave-shaped layers. Within the layers, there are strong H-bonds. Indeed, the spectral changes of NH2 (νas) at 3443 cm-1 are attributed to the restructing of the H-bonding network in the α→β phase transition[4]. The C=C and amino nitrogen atoms form a molecular plane, and the nitro oxygen atoms deviate strongly from the molecular plane. The results have been previously reported[8]that the NO2 twist angle does not vary significantly on heating up to 100 ℃, but it increases abruptly above 100 ℃. The unique spectra signature of ν10 is likely associated with changes of the NO2 twist angle when the temperature increases. The analytical results of the IR spectra indicate that intermolecular hydrogen bonding interactions change in the phase transitions of the FOX-7, which cause the changes of the molecular structure. The results confirm that the α→β is a displacive transition with minor structural distortions[8].
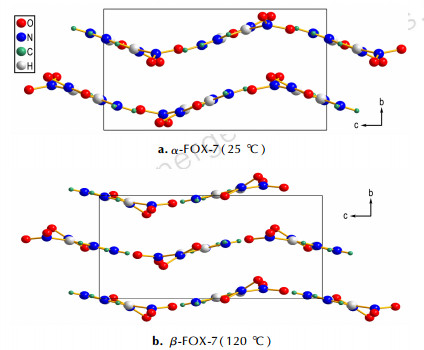
|
Fig.2 View along a axis of α-FOX-7 at 25 ℃ and β-FOX-7 at 120 ℃ |
Most materials experience relatively modest expansions with increasing temperature, resulting from the increasing anharmonic vibration amplitudes of the ingredient atoms or molecules[26]. The degree of linear thermal expansion has been quantified using a coefficient defined as α=(lT-l0)/(l0(T-T0))[27], where lT is the axis length at the final temperature, and l0 is the axis length at the initial temperature. The average linear thermal expansion coefficients of α-FOX-7 are αa=2.18×10-5 ℃-1, αb=12.71×10-5 ℃-1 and αc=4.29×10-5 ℃-1. The average linear thermal expansion coefficients of β-FOX-7 are αa=0.84×10-5 ℃-1, αb=16.89×10-5 ℃-1 and αc=3.85×10-5 ℃-1 [28]. Similarly, the H-bonding length is formulated as lT= l0+αΔT[27], where lT is the H-bonding length at final temperature, l0 is the H-bonding length at initial temperature, α is the coefficient of thermal expansion, and ΔT is the change of temperature. Here, the value of α for α- and β-FOX is almost equal to 1×10-5 ℃-1. The intermolecular H-bonds of FOX-7 at 25 ℃ and 120 ℃ are presented in Fig. 3, and their length are displayed in Table 2. The average H-bonds length between FOX-7 molecules is 2.58 Å at 25 ℃ and 2.76 Å at 120 ℃. We find that l0+αΔT=(2.58+1×10-5×95) Å≈2.58 Å= l0 ≠ lT=2.76 Å. As described in Section 3.1.1, the length of H-bonding remains constant during heating from 55 to 100 ℃. We conclude that a intermediate ltrans occurs in the α→β transition, as is followed: lT= l0+αΔT+ ltrans, where lT is the H-bonding length at final temperature, l0 is the H-bonding length at initial temperature, α is the coefficient of thermal expansion, ΔT is the change of temperature, ltrans is the H-bonding length increamental resulting from the phase transition and increase gradually when the temperature increases from 100 ℃ to 110 ℃. Besides, the evolution of selected peaks frequencies with increasing temperature is presented. As shown in Fig. 4, the frequencies of FOX-7 in all vibrational bands show abrupt changes during heating in the range of 100 ℃ to 110 ℃. Three or more points are observed in the range of 100 ℃ to 110 ℃, and each one of them is neither α-FOX-7 nor β-FOX-7, which giving more evidence of the occurrence of a transition state in the phase transition. It is the transition state that makes the change of H-bonding length with temperatures, not consistent with the formula (lT= l0+αΔT). The intermediate should be a necessary step in the transition from α-FOX-7 to β-FOX-7. Traditionally, phase transition is considered to be one-step reaction.
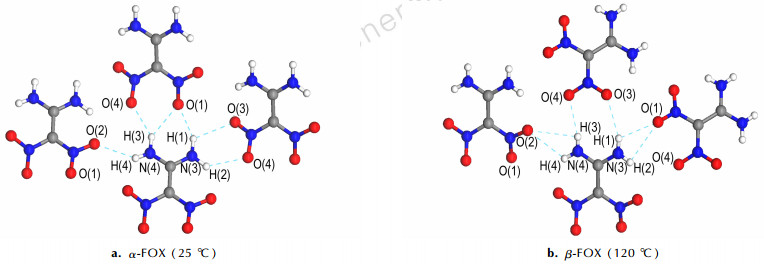
|
Fig.3 H-bonding interactions of FOX-7 |
| Tab.2 H-bonds length between FOX-7 molecules at 25 ℃ and 120 ℃ |

|
Fig.4 Peak positions of different vibrational modes as a function of temperature |
With further application of temperature to 190 ℃, spectra were acquired and displayed in Fig. 5. In β-phase, the two NH2 (νas) peaks are well separated and dramatically shifted to higher frequencies. As temperature increases, the two peaks are broad and strongly overlapped with each other in the range of 3500-3350 cm-1 as well as being accompanied by the loss of NH2 (νs) modes at~3329 cm-1. NO2-related vibrations such as ν12 and ν20 exhibit significant changes, indicating that the γ transition was obtained. Compared to the changes observed in the α→β transition, the frequencies of most vibrational bands remain nearly constant in the β→γ transition, implying no further weakening of the H-bonding network. The changes in NO2-related vibrations are smaller during the β→γ transition than the α→β transition. The previous results[9] showed that the displacement of the nitro groups with respect to the molecular plane remains invariant in β-and γ-FOX-7. FTIR spectra of FOX-7 exhibit minor changes in the β→γ transition. A combination of stable H-bonding network and structural similarities is responsible for subtle differences in the FTIR spectra for two polymorphs over the whole temperature range from 122 ℃ to 190 ℃.
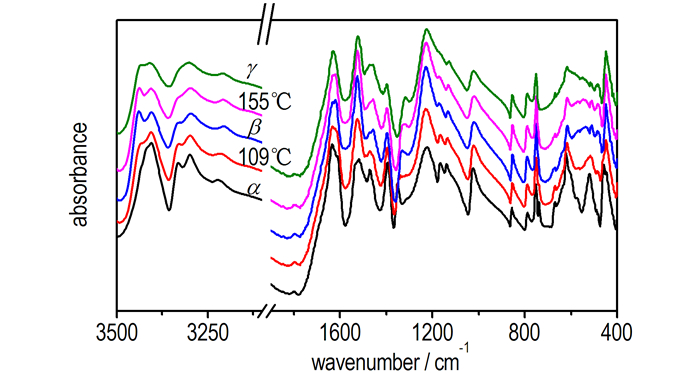
|
Fig.5 FTIR spectra of FOX-7 at five different temperatures (α-FOX-7 at 55 ℃; β-FOX-7 at 122 ℃; γ-FOX-7 at 190 ℃) |
Standard FTIR spectra of α, β and γ polymorphs of FOX-7 are identified and confirmed by X-ray diffraction patterns (see in Fig. 6). The FTIR spectra of α-, β-and γ-FOX-7 exhibit significant changes including NH2 and NO2-related vibrations such as ν12, ν18, ν20 and ν21. The former is due to the restructing of the H-bonding network, and the latter is due to the changes in the displacement of nitro groups with respect to the molecular plane. The characteristic FTIR absorption bands of each phase of FOX-7 are presented in Table 3.

|
Fig.6 XRD patterns of FOX-7 at five different temperatures (α-FOX-7 at 55 ℃; β-FOX-7 at 122 ℃, γ-FOX-7 at 190 ℃) |
| Tab.3 The characteristic FTIR absorption bands of each phase of FOX-7 |
Temperature-dependent FTIR spectroscopy has been employed to study the phase transitions of FOX-7 in real time. The main conclusions are as follows:
(1) In the process of phase transitions of the FOX-7, intermolecular hydrogen bonding interactions change, which caused the changes of the molecular structure.
(2) The transition state was confirmed by the special thermal expansion properties of H-bonding length and the special frequencies of ν1′, ν4[Fermi resonance between the NH2(νas) and the overtone mode of the NH2 scissor(2ν5)], ν7 and ν10[NO2(νas)] in the α→β transition with increasing temperature. α-FOX-7 does not directly transforms to β-FOX-7, the transition state should be a necessary step.
(3) Standard FTIR spectra of α, β and γ-FOX-7 were identified and confirmed in-situ by powder X-ray diffraction (XRD). Several characteristic peaks of ν12[C—NH2(ν)], ν18[C—NO2(ν)] and ν20 [NO2(ω)] can be used to identify each phase of FOX-7.
| [1] |
Latypov N V, Bergman J, Langlet A, et al. Synthesis and reactions of 1, 1-diamino-2, 2-dinitroethylene[J]. Tetrahedron, 1998, 54: 11525-11536. DOI:10.1016/S0040-4020(98)00673-5 |
| [2] |
Östmark H, Langlet A, Bergman H, et al. Report No[J]. ONR 3330-5, Office of Naval Research, Arlington, V A, 2000, 807-808. |
| [3] |
Bemm U, Eriksson L. Phase Transitions in FOX-7[C]//Proc Insens Muni Energ Mater Tech Symp, 2001: 775-790.
|
| [4] |
Bishop M M, Chellappa R S, Pravica M, et al. 1, 1-diamino-2, 2-dinitroethylene under high pressure-temperature[J]. J Chem Phys, 2012, 137(17): 10870-10870. |
| [5] |
Li J, Thomas B Brill. Kinetics of solid polymorphic phase transitions of CL-20[J]. Propel, Explo, Pyrotech, 2007, 32: 326-330. DOI:10.1002/(ISSN)1521-4087 |
| [6] |
Kempa P B, Herrmann M. Temperature resolved X-ray diffraction for the investigation of the phase transitions of FOX-7[J]. Part Part Syst Charact, 2005, 22(6): 418-422. DOI:10.1002/ppsc.v22:6 |
| [7] |
Evers J, Klapötke T M, Mayer P. α-and β-FOX-7 polymorphs of a high energy density material studied by X-ray single crystal and powder investigations in the temperature range from 200 K to 423 K[J]. Inorg Chem, 2006, 45(13): 4996-5007. DOI:10.1021/ic052150m |
| [8] |
Crawford M J, Evers J, Göbel M, et al. γ-FOX-7: structure of a high energy density material immediately prior to decomposition[J]. Propel Explo Pyrotech, 2007, 32(6): 478-495. DOI:10.1002/(ISSN)1521-4087 |
| [9] |
Dreger Z A, Tao Y, Gupta Y M. Polymorphs of 1, 1-diamino-2, 2-dinitroethene (FOX-7): Isothermal compression versus isobaric heating[J]. Chem Phys Lett, 2013, 584: 83-87. DOI:10.1016/j.cplett.2013.08.070 |
| [10] |
Pravica M, Liu Y, Robinson J, et al. A high-pressure far-and mid-infrared study of 1, 1-diamino-2, 2-dinitroethylene[J]. J Appl Phys, 2012, 111(10): 103534-103537. DOI:10.1063/1.4722350 |
| [11] |
Peiris S, Wong C, Kuklja M, et al. Equation of State and Structural Changes in Diaminodinitroethylene from Experimental Studies and Ab-Initio Quantum Calculations[C]//In 12th Int Deton Symp Proc, 2002, 120617-120624.
|
| [12] |
Zhao J, Liu H. High-pressure behavior of crystalline FOX-7 by density functional theory calculations[J]. Comp Mater Sci, 2008, 42: 698-703. DOI:10.1016/j.commatsci.2007.10.008 |
| [13] |
LI Jing-you, ZHANG Hao-bin, XU Jin-jiang, et al. IR absorption peaksassignments of LLM-105 by temperature-dependent FT-IR spectroscopy[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2015, 23(5): 507-510. |
| [14] |
Pan Q, Deng L. Some key techniques of measuring propellants and explosives by temperature-dependent FTIR[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2007, 15(6): 676-680. |
| [15] |
ZHANG Zai-juan, LUO Yun-jun, LI Guo-ping. Reaction kinetics of GAP and three kinds of isocyanates with variable temperature FTIR spectrum method[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(3): 382-385. |
| [16] |
Bishop M M, Chellappa R S, Liu Z, et al. High pressure-temperature polymorphism of 1, 1-diamino-2, 2-dinitroethylene[C]//Journal of Physics: Conference Series IOP Publishing, 2014, 500(5): 052005-052007.
|
| [17] |
Sui H L, Zhong F C, Cheng K M, et al. IR vibrational assignments for 1, 3, 5-triamine-2, 4, 6-trinitrobenzene (TATB) based on the temperature-dependent frequency shifts[J]. Spectrochim Acta A, 2013, 114: 137-143. DOI:10.1016/j.saa.2013.05.067 |
| [18] |
Socrates G. Infrared and Raman characteristic group frequencies: tables and charts[M]. John Wiley & Sons, 2004.
|
| [19] |
Dheivamalar S, Silambarasan V. DFT simulations and vibrational analysis of FTIR and FT-Raman spectra of 2-amino-4-methyl benzonitrile[J]. Spectrochim Acta A, 2012, 96: 480-484. DOI:10.1016/j.saa.2012.05.008 |
| [20] |
Sathyanarayana D N. Vibrational Spectroscopy-Theory and Applications[M]. Second ed., New Age International (P) Limited Publishers, New Delhi, 2004.
|
| [21] |
Sundaraganesan N, Meganathan C, Kurt M. Molecular structure and vibrational spectra of 2-amino-5-methyl pyridine and 2-amino-6-methyl pyridine by density functional methods[J]. J Mol Struc, 2008, 891(1): 284-291. |
| [22] |
Silverstein M, Clayton G, Basseler, Moril C. Spectro Metric Identification of Organic Compounds[M]. New York: Wiley, 1981.
|
| [23] |
Thilagavathi G, Arivazhagan M. Density functional theory calculation and vibrational spectroscopy study of 2-amino-4, 6-dimethyl pyrimidine (ADMP)[J]. Spectrochim Acta A, 2011, 79(3): 389-395. DOI:10.1016/j.saa.2011.01.052 |
| [24] |
Clarkson J, Ewen Smith WA. DFT analysis of the vibrational spectra of nitrobenzene[J]. J Mol Struc, 2003, 655(3): 413-422. DOI:10.1016/S0022-2860(03)00316-8 |
| [25] |
Hess G.. Tunable fermi resonance in a C2F6C2F6 monolayer on graphite[J]. J Chem Phys, 2002, 116(15): 6777-6781. DOI:10.1063/1.1462611 |
| [26] |
Miller W, Smith C, Mackenzie D, et al. Negative thermal expansion: a review[J]. J Mater Sci, 2009, 44(20): 5441-5451. DOI:10.1007/s10853-009-3692-4 |
| [27] |
Engel E R, Smith V J, Bezuidenhout C X. Uniaxial negative thermal expansion facilitated by weak host-guest interactions[J]. Chem Comm, 2014, 50(32): 4238-4241. DOI:10.1039/C4CC00849A |
| [28] |
Qian W, Zhang C Y, Zong H H, et al. Simulation study on the anisotropy of thermal expansion for crystalline 1, 1-diamino-2, 2-dinitroethylene[J]. Chin J Atomic and Mol Phys, 2014, 31(3): 454-462. |

Two kinds of phase transitions (α→β and β→γ) of 1, 1-diamino-2, 2-dinitroethylene (FOX-7) have been investigated by temperature-dependent Fourier transform infrared (FTIR) spectroscopy, and the effects of intermolecular H-bonding interactions and molecular structure in the phase transitions of FOX-7 were discussed.




