2. College of Safety Science and Engineering, Nanjing Tech University, Nanjing 211800, China;
3. School of Chemical Engineering, Nanjing University of Science & Technology, Nanjing 210094, China;
4. School of Pharmacy, China Pharmaceutical University, Nanjing 210009, China
2. 南京工业大学安全科学与工程学院, 江苏 南京 211800;
3. 南京理工大学化工学院, 江苏 南京 210094;
4. 中国药科大学药学院, 江苏 南京 210009
Polyamino and polynitro derivatives of pyridine are good examples of a general structure-property relationship among energetic ingredients, that the addition of amino-groups to a polynitropyridine increases the density and thermal stability and decreases the sensitivity compared to the corresponding H-atom-substituted nitropyridine. In general, the density increase outweighs the concomitant decrease in oxygen balance and heat of formation that accompanies the introduction of amino groups, resulting in better performance. Also, the decrease in oxygen balance and heat of formation, along with increased hydrogen bonding between the amino group and the nitro-groups, decreases sensitivity and increases thermal stability[1-2]. However, it is obvious that most energetic materials mentioned above have the disadvantage of detonation performance due to their low heat of formation and poor oxygen balance, especially detonation velocity (D) and detonation pressure (p). One approach to improve this insufficiency is to basically incorporate maximum possible percentage of nitrogen into energetic materials, because the introduction of nitrogen-rich energetic blocks into the nitropyridine determines some unique properties such as heat of formation, significant gas release in the form of environmentally benign nitrogen, and commonly include moieties such as five membered azoles[3-5].
Based on our continuous effort to synthesize insensitive high explosives, we have successfully synthesized several energetic four-substituted energetic pyridine derivatives such as 2-(5-amino-1H-tetrazol-2-yl)-4-amino-3, 5-dinitropyridine and 6-(5-amino-1H-tetrazol-2-yl)-2-amino-3, 5-dinitropyridine, whose detonation velocity are both 8.18 km·s-1 and detonation pressure are 30.7 GPa[4]. In the pursuit of new fully substituted energetic pyridine derivative, we have recently been focus on the synthesis and characterization of 4-amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine(ABDP), as well as the thermal performance was studied by thermogravimetry and differential scanning calorimetry.
2 Experimental 2.1 Materials and InstrumentsAll chemicals used in the present study were of AR grade and purchased from the trade without further purification. Melting point was measured on a X-4 melting point apparatus and was uncorrected. 1H NMR and 13C NMR were recorded on a Bruker Avance Spectrometer. The coupling constants (J) were reported in hertz (Hz). High-resolution mass spectra were recorded on a Finnigan TSQ Quantum ultra AM mass spectrometer. Elemental analyses were carried out on a Perkin-Elmer instrument. Thermogravimetry and differential scanning calorimetry (TG-DSC) analysis was conducted on a Q600SDT.
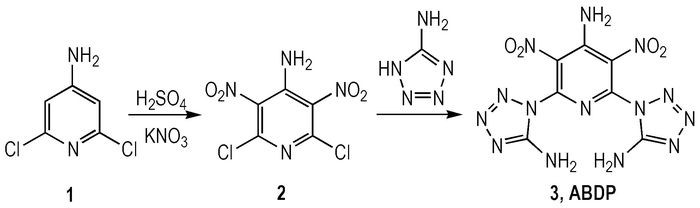
2.2 Synthetic RouteThe synthetic route of ABDP is shown in Scheme 1.
|
Scheme1 The synthetic route of ABDP |
4-Amino-2, 6-dichloropyridine (1) (2.04 g, 12.59 mmol) was dissolved in 20 mL of concentrated sulfuric acid at room temperature, and potassium nitrate (3.40 g, 33.66 mmol) was added in portions with vigorous stirring. After the solution was clear, the reaction mixture was heated to 50 ℃ for 7 h and then cooled to room temperature. After pouring over ice, the solid was precipitated, filtered, washed with cold water, and then dried to give 4-amino-2, 6-dichloro-3, 5-dinitropyridine (2) as a yellow solid (1.91 g, 60%), m.p. 158-160 ℃. 1H NMR (DMSO-d6, 500 MHz): 8.25(s, 2H); 13C NMR (DMSO-d6, 125 MHz): 142.39, 141.03, 132.00; Anal. Calcd. for C5H2Cl2N4O4: C 23.74, H 0.80, N 22.14; found: C 23.65, H 1.02; N 22.19; MS (ESI) m/z: 250.82:252.83:254.82=9:6:1 (M-H).
2.4 Synthesis DescriptionTo a stirred solution of 5-amino-1H-tetrazole (0.85 g, 10 mmol) in 30 mL ethanol was added potassium bicarbonate (0.50 g, 5 mmol) and tetrabutylammonium bromide (0.16 g, 0.5 mmol) portionwise. The resulting mixture was kept at room temperature for 30 min, then 4-amino-2, 6-dichloro-3, 5-dinitropyridine(1.26 g, 5 mmol)was added. The reaction mixture was heated to 40 ℃ for 15 min and then cooled to room temperature. After pouring over ice, the solid was precipitated, filtered, washed with methanol and water for three times, and then dried to give a yellow solid 4-amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine (3, ABDP) (1.06 g, 61%); 1H NMR (DMSO-d6, 400 MHz): 12.01(s, 2H), 10.14(s, 2H); 13C NMR (DMSO-d6, 100 MHz): 152.47, 150.82, 148.87, 112.19; Anal. Calcd. for C7H6N14O4: C 24.01, H 1.73, N 55.99; found: C 24.10, H 1.82, N 56.11; MS (ESI)m/z: 351.12(M+H).
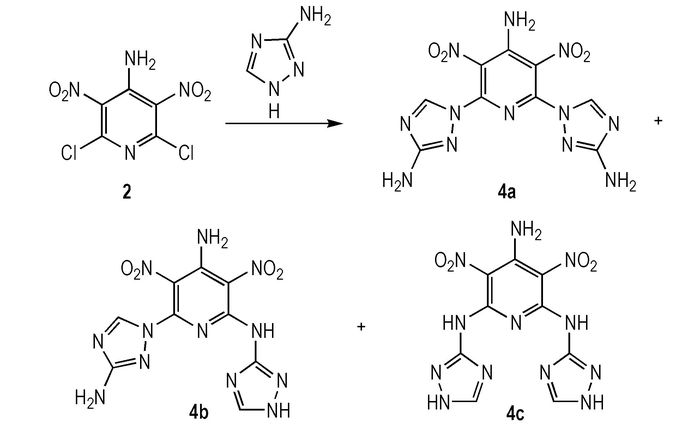
3 Results and Discussion 3.1 Synthesis of 3By investigation of the successful synthesis of 2-(5-amino-2H-tetrazol-2-yl)-4-amino-3, 5-dinitropyridine using 2-chloro-4-aminopyridine as starting material, fully substituted pyridine derivatives were designed by combining nitrogen rich compounds with orth-amino and nitro pyridines, so as to improve energetic properties but remaining insensitive performance. 4-nitroimidazole, nitroguanidine, 3-amino-1, 2, 4-triazole, 5-amino-1H-tetrazole were attempted to react with 4-amino-2, 6-dichloro-3, 5-dinitropyridine (2, ADDP) under appropriate conditions. However, the former two compounds failed to obtain the ideal products, mainly due to their weak nucleophilicity. When 3-amino-1, 2, 4-triazole was reacted with ADDP, three peaks were detected by liquid chromatograph/mass spectrometer (LC/MS), but only to find the same molecular mass (MS (ESI) m/z: 349.3 (M+H)) (Scheme 2). It is difficult to separate these three substances with regular column chromatography because of their poor solubility and polarity. Intermediates were also studied to find two products with the same molecular mass (MS (ESI) m/z: 301.2 (M+H)) (Scheme 3). This phenomenon maybe prove similar nucleophilicity for primary amine and secondary amine of 3-amino-1, 2, 4-triazole.
|
Scheme2 Synthesis of 3-amino-1, 2, 4-triazole substituted pyridines |

|
Scheme3 Synthesis of 3-amino-1, 2, 4-triazole substituted pyridines |
When reacted with 4-amino-2, 6-dichloropyridine, 5-amino-1H-tetrazole was rapidly converted into 4-amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine (3, ABDP) at 40 ℃, and mass spectrum showed that its sole molecular mass was 350, 1H NMR spectrum showed that two hydrogen signal in the amino group attaching to the pyridine ring was at 12.01 and one of the two amino groups attaching to the tetrazole ring was at 10.14, but the other was exchanged in the solution. The same pheonomenon occurs in the synthesis of 6-(5-amino-1H-tetrazol-2-yl)-2-amino-3, 5-dinitropyridine[6] whose structure is similar to that of ABDP.
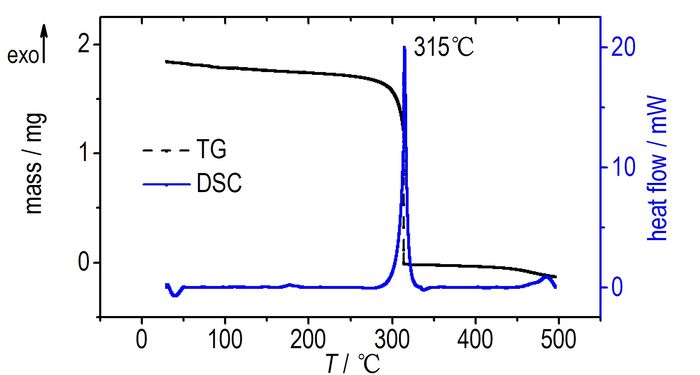
3.2 Thermal PerformanceThe TG-DSC analysis was carried out at a heating rate of 5 ℃·min-1 from 50 ℃ to 500 ℃ under N2 atmosphere. The TG-DSC curves were showed in Fig. 1, which revealed that 4-amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine (3, ABDP) was thermally stable up to 315 ℃ and possessed a direct thermal decomposition process with a mass loss of 94%.
|
Fig.1 TG-DSC curves of ABDP |
Detonation velocity (D), detonation pressure (p) and oxygen balance are calculated according to Rothstein′s method[6-7]. For the ideal C, H, N, O type explosives, detonation velocity was predicted from the linear regression equation (1) :
| $D = \frac{{F - 0.26}}{{0.55}}$ | (1) |
While the factor F is expressed as equation (2) :
| $F = \left[ {100 \times \frac{{n\left( {\rm{O}} \right) + n\left( {\rm{N}} \right) - \frac{{n\left( {\rm{H}} \right)}}{{2n\left( {\rm{O}} \right)}} + \frac{A}{3} - \frac{{n\left( {\rm{B}} \right)}}{{1.75}} - \frac{{n\left( {\rm{C}} \right)}}{{2.5}} - \frac{{n\left( {\rm{D}} \right)}}{4} - \frac{{n\left( {\rm{E}} \right)}}{5}}}{M}} \right] - G$ | (2) |
Where G=0.4 for liquid explosives, for solid explosives G=0; A=1 if the compound is aromatic, otherwise A=0 and where for one mole of the composition: n(O)= number of oxygen atoms, n(N)= number of nitrogen atoms, n(H)= number of hydrogen atoms, n(B)= number of oxygen atoms in excess of those already available to form CO2 and H2O, n(C)= number of oxygen atoms doubly bonded directly to carbon as in carbonyl 

Detonation pressure was predicted according to the equation (3) :
| $p = \frac{{93.3D - 456}}{{10}}$ | (3) |
Some detonation performance of ABDP are listed in Table 1, and compared with some known thermally stable energetic materials: hexanitrostibene(HNS), tetranitrodibenzo-1, 3α, 4, 6α-tetrazapentalene(TACOT), 1, 3, 5-triamino-2, 4, 6-trinitropyridine (TATB), 2, 6-bis(picrylamino)-3, 5-dinitropyridine(PYX).
| Tab.1 Detonation performance of compounds ABDP, TACOT, TATB, HNS and PYX |
It was found that the oxygen balance of 4-amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine is calculated to be -59.4%, and this value is lower than that of PYX(-55.4%) and TATB(-55.8%), but higher than that of HNS(-67.5%) and TACOT(-77.9%). All thermally stable energetic materials mentioned in Table 1 exhibit a melting or decomposition temperature above 310 ℃. Furthermore, ABDP has a detonation velocity of 8823 m·s-1 and a detonation pressure of 36.72 GPa, which are significantly better than those of PYX, HNS, TACOT and TATB, revealing a better detonation performance as a novel potential thermally stable energetic material.
4 Conclusions(1) 4-Amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine was first synthesized via nitration and condensation using 4-amino-2, 6-dichloropyridine as a raw material with a total yield of 36%. Its structure was characterized by nuclear magnetic resonance (NMR), mass spectrum (MS) and elemental analysis.
(2) ABDP exhibits some good detonation properties, such as high detonation pressure (36.72 GPa) and detonation velocity (8823 m·s-1).
(3) TG-DSC curves indicate that ABDP has a thermal decomposition peak at 323 ℃ accompanied with a mass loss of 97%.
| [1] |
Badgujar D M, Talawar M B, Asthana S N, et al. Advances in science and technology of modern energetic materials: an overview[J]. Journal of Hazardous Materials, 2008, 151(2-3): 289-305. DOI:10.1016/j.jhazmat.2007.10.039 |
| [2] |
Sikder A K, Sikder N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications[J]. Journal of Hazardous Materials, 2004, 112(1): 1-15. |
| [3] |
Klapötke T M, Stierstorfer J, Wallek A U. Nitrogen-rich salts of 1-methyl-5-nitriminotetrazolate: an auspicious class of thermally stable energetic materials[J]. Chemistry of Materials, 2008, 20(13): 4519-4530. DOI:10.1021/cm8004166 |
| [4] |
Tao G H, Twamley B, Jean′ne M S. A thermally stable nitrogen-rich energetic material-3, 4, 5-triamino-1-tetrazolyl-1, 2, 4-triazole (TATT)[J]. Journal of Materials Chemistry, 2009, 19(32): 5850-5854. DOI:10.1039/b908214j |
| [5] |
Kumar A S, Ghule V D, Subrahmanyam S, et al. Synthesis of thermally stable energetic 1, 2, 3-triazole derivatives[J]. Chemistry-A European Journal, 2013, 19(2): 509-518. DOI:10.1002/chem.201203192 |
| [6] |
ZHAO Kun, LIU Zu-liang, MA Cong-ming. Synthesis and performance of two new 1-substituted 5-aminotetrazole energetic derivatives[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2015, 23(11): 1099-1102. |
| [7] |
Rothstein L R, Petersen R. Predicting high explosive detonation velocities from their composition and structure[J]. Propellants, Explosives, Pyrotechnics, 1979, 4(3): 56-60. DOI:10.1002/(ISSN)1521-4087 |
| [8] |
ZHOU Jie-wen, ZHANG Chuang-jun, WANG You-bing, et al. Status and research progress of heat resistant explosives[J]. Journal of Ordnance Equipment Engineering, 2016, 37(3): 111-115. |
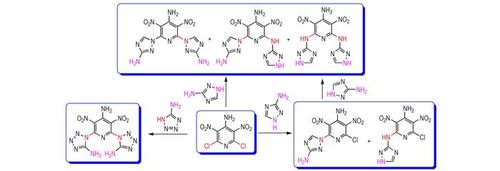
4-Amino-2, 6-bis(5-amino-1H-tetrazol)-3, 5-dinitropyridine was synthesized by the reaction between 5-amino-1H-tetrazole and 4-amino-2, 6-dichloro-3, 5-dinitropyridine(ADDP). However, when 3-amino-1, 2, 4-triazole was reacted with ADDP, two or three intermediates with the same molecular mass were obtained, which were hard to isolate and purify.




