2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China
2. 西安近代化学研究所, 陕西 西安 710065
As we all known that the high-nitrogen energetic compounds derive most of their energy from the very large positive enthalpy of formation rather than from oxidation of the fuel like carbon backbone. The materials are particularly suitable for consideration in high-performance propellant applications, because of their high positive enthalpy of formation, insensitivity to impact, friction and electrostatic discharge, and low-molecular-weight reaction products.
3, 6-Bis(1 H-1, 2, 3, 4-tetrazol-5-yl-amino)-s-tetrazine(BTATz) is one of the high-nitrogen energetic compounds, with nitrogen content of 79.02%, density of 1.76 g·cm-3, enthalpy of formation of +883 kJ·mol-1, and moderate mechanical sensitivity [1-6]. BTATz was first synthesized by Hiskey at Los Alamos National Laboratory [7], then the detailed studies have been done, such as the purification and the quantum chemistry study of BTATz [4], the burning rate measurement [8], thedissolution properties in N-methyl pyrrolidone and dimethyl sulfoxide [9], its metal complexes and metal salts [10-14], even as a substitute of hexogen (RDX) in the composite modified double base (CMDB) propellant formulation [15] and the decomposition reaction kinetics and thermal safety of BTATz-HNIW-CMDB propellants [16]. Many researches focused on the BTATz show that it has a prospect using as a primary component in the high burning rate propellant for the booster rocket motor and the kinetic energy ammunition, and it also can be used in the minimum signature propellant for the smokeless ammunition [14, 17-19].
Generally, energetic nitrogen rich salts become the most exciting developments and continue to attract more interesting than atomically similar non-ionic analogues, due to the excellent performance of lower vapour pressures, higher heats of formation, and better thermal stability [20]. Salt formation of the acidic precursor by direct neutralization or metathesis reactions with alkaline nitrogen-rich cations is a very effective method to increase the nitrogen content, the heats of formation and the possibility to form the hydrogen bond, as a consequence of the densities and performances. Therefore, some energetic ionic salts have been reported [21-23]. For BTATz, its di-ammonium, di-hydroxylammonium, and di-hydrazinium salts had been synthesized and added into composite propellant [2, 19], and the result indicates that all of them have the potential to be a propellant component.
Herein, three novel energetic ionic salts based on BTATz (Scheme 1) with excellent physical chemistry properties were first presented with BTATz and different amine salts in the solvent of DMF. The structure of salts has been fully characterized. The differential scanning calorimetry(DSC) and thermogravimentry(TG) technique were adopted to investigate the thermal behavior at 300-800 K. Theoretical calculations predicting energetic performances were also carried out.
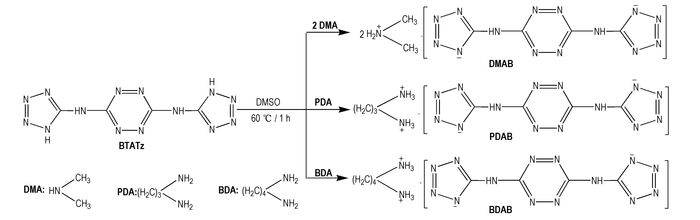
|
Scheme1 Synthesis of three energetic ionic salts based on BTATz |
BTATz used in this work was synthesized according to the literature method [4]. All solvents were obtained from typical commercial sources and used without further treatment.
2.2 Experimental Equipments and ConditionsA suitable crystal of PDAB was selected and its crystal structure was determined bya Bruker SMART APEXⅡ diffractometer. X-ray diffractometer using MoKa radition (λ=0.071073 nm) graphite monochromatization. The crystal structure was solved by direct methods (SHELXTL-97), the data were revised with Lp and empirical absorption, and F2 was corrected with complete matrix least-squares procedure [24].
The TG and DSC curves under the condition of flowing nitrogen gas (purity, 99.999%; atmospheric pressure) were obtained by using a TA2950 thermal analyzer (TA Co., USA) and a 204HP differential scanning calorimeter (Netzsch Co., Germany). The sample mass was about 1mg at the heating rate (β) of 10 K·min-1 to TG-DTG experiments and the N2 flowing rate was 40 cm3·min-1; for DSC analyses, the N2 flowing rate was 50 cm3·min-1, the Al2O3 was used as reference sample and the type of crucible was aluminum pan with a pierced lid. The sample mass was about 1mg at different heating rate of 5, 10, 15 and 20 K·min-1, respectively. The specific heat capacity (cp, J·g-1 ·K-1) was determined with continuous cp mode on a Micro-DSC Ⅲ mircocalorimeter (Setaram Co., France) in the atmosphere of N2, the reference sample was calcined a-Al2O3 and the sample mass was about 100 mg with the heating rate of 0.15 K·min-1.
2.3 Synthesis and Characterization of the Salts of BTATzThe salts of BTATz were prepared via one-pot method (Scheme 1). In brief, aqueous dimethylamine (33%) /1, 3-propanediamine/1, 4-butanediamine ethylenediamine (equivalent molar to BTATz) was dropwise added to 20 mL DMSO contained 2 mmol BTATz under magnetic stirring at room temperature. Then, the mixtures were heated to 333.15 K under vigorous stirring for 1 h. Upon the completion of the reaction, the red precipitate was filtered off, washed with a copious amount of ethanol to remove excessive original materials, and dried at 333.15 K at a vacuum drying oven for 3 h. The resulting compounds were donated as DMAB (dimethylaminium salt of BTATz), PDAB (1, 3-propanediaminium salt of BTATz) and BDAB (1, 4-butanediaminium salt of BTATz), respectively.
DMAB: yeild 49.5%; IR(KBr, ν/cm-1): 3241(—NH2), 3014 (N—H), 2853, 2766 (—CH3), 1741, 1608 (C—N), 1434 (N—N), 1046, 965, 771; elemental analysis (%) calcd for C8N16H18: C 28.76, H 3.857, N 63.58; found: C 28.41, H 5.340, N 65.13; 1H NMR (DMSO-d6, 400 Hz) δ: 2.35 (d, J=18.82, —CH3), 2.59-3.05 (m, —NH—CH3), 3.34 (s, —NH(BTATz)); 13C NMR (DMSO-d6, 400 Hz) δ: 34.51 (s), 157.27 (s), 159.36 (s).
PDAB: yield 72.5%. IR (KBr, ν/cm-1): 3435 (—NH2), 3248 (N—H), 2967, 2793(—CH2—), 1601, 1501(C—N), 1434 (N—N), 1046, 959, 765; elemental analysis (%) calcd for C7N16H14: C 26.03, H 3.230, N 65.32; found: C 25.97, H 3.641, N 63.77; 1H NMR (DMSO-d6, 600 Hz) δ: 1.66-2.02 (m, —CH2—CH2—NH2), 2.60-2.64 (m, —CH2—NH2), 2.99 (t, J=7.3Hz, —NH2), 3.33 (s, —NH(BTATz)).
BDAB: yield 73.6%. IR(KBr, ν/cm-1): 3222(—NH2), 2853 (—CH2—), 2144 (—NH3), 1655, 1581, 1494 (C—N), 1427 (N—N), 1052, 959, 724; elemental analysis(%) calcd for C8N16H16: C 29.39, H 3.771, N 61.93; found: C 28.63, H 5.061, N 63.05; 1H NMR(DMSO-d6, 600 Hz) δ: 1.48-1.72(m, —CH2—CH2—NH2), 2.63-2.93(m, —CH2—NH2), 2.93-3.59(m, —NH2), 3.59(s, —NH(BTATz)).
All salts of the BTATz, which were isolated as powder materials, are non-hygroscopic and stable in air. The single crystal of PDAB suitable for X-ray determination was obtained by slow evaporation of the deionized water at room temperature. PDAB crystallizes in monoclinic C2/ c and crystal parameters of a=2.2699(10) nm, b=0.5098(2) nm, c=1.6449(6) nm, β=93.045(15)°, V=1.9008(13) nm3, Dc=1.504 g·cm-3, Z=4, F(000)=912, μ=0.127 mm-1, R1=0.0673, wR2=0.2002. The main bond length and angle are listed in Table 1, and its structure is shown in Fig. 1a. The crystal data have been deposited in CCDC with the number of 823528.
| Tab.1 Selected bond length and angles for the PDAB |
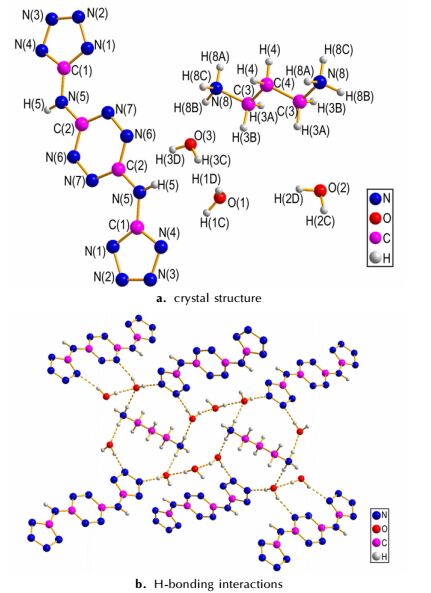
|
Fig.1 Crystal structure and interaction of the intermolecular hydrogen bonds for PDAB |
The results of the elemental analysis are a bit of different from the crystal structure, because the measurement state is different between two methods. The solid powder was used in the elemental analysis, while the single structure that is suitable to X-ray diffract obtained from the solvent. The analytical results indicate that there are a cation C3N2H122+(1, 3-propanediamine), an anion C4N14H22-(BTATz2-), and three crystal water molecules in one unit.
The average bond lengths of C—N (0.1338 nm) and N—N (0.1331 nm) in tetrazine ring are shorter than the normal length of C—N (0.1450 nm), which implies the existence of conjugated action in tetrazine ring and the tetrazole ring. BTATz anion consisted by the atoms of N(1)—N(7), C(1) and C(2) shows the good coplanarity, its plane equation is as following: 15.309 x-3.196 y+5.819 z=3.0282.
There are intermolecular N—H…O hydrogen bonds linking four water molecules, andN atom of BTATz interact to O atom of H2O by the hydrogen bond O(2)—H(2D)…N(2) and O(1)—H(1D)…N(3). The data of hydrogen bond are shown in Table 2. Two BTATz molecules, one 1, 3-propanediamine molecule and six free water molecules link by the hydrogen bond to form a ring structure. These rings are in turn linked by the intermolecular O(2)—H(2C)…O(3) and O(3)—H(3C)…O(1) hydrogen bonds to form the two-dimensional chain structure, which is shown in Fig. 1b.
| Tab.2 The hydrogen bond data for PDAB |
The thermal behavior and decomposition character of the BTATz salts are determined by DSC and TG. Typical DSC-TG curves obtained are shown in Fig. 2.
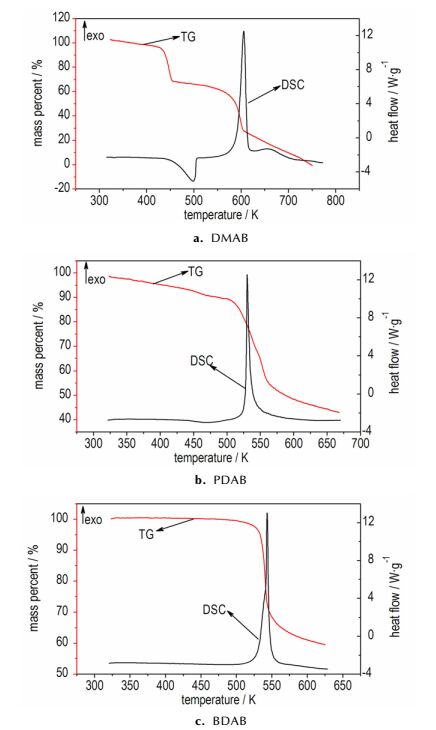
|
Fig.2 DSC and TG curves for different samples at a heating rate of 10 K·min-1 |
Fig. 2a shows that there is one endothermic (435.15-507.05 K) and two exothermic processes (547.05-624.75 K, 630.25-712.65 K) in DSC curve for DMAB, which is corresponding to the two mass loss stages in TG curve. It can be inferred that the melting process overlaps with the thermal decomposition one leading to a one-step mass loss (29.20%) according to our results in Fig. 2a. A mass loss of 29.20% can be regard as the breaking of the ion bond between two N atoms and the losing of two dimethyamine ions from the system, which is near to the theory one (27.20%). The big exothermic process should be caused by the breaking of the C—N bond that connected the tetrazole and the tetrazine, and the mass loss can be identified with the loss of two tetrazole rings (63.51%), which is close to the theoretical value (64.49%).
Notably, the DSC and TG curves for the other two salts are similar to each other (Fig. 2b and Fig. 2c). There is only one exothermic process in DSC curve and one mass loss stage in TG curve. The thermal decomposition process occurs at the temperature range of 507.85-555.35 K for PDAB and 494.85-583.95 K for DBAB, the peak temperature is 530.05 K and 542.77 K, respectively. Both of them are corresponding to the mass loss of 57.23% and 34.20% in the TG curves. As a consequence, BDAP should be the ring opening of the tetrazine and the break of ion bond (theoretical value 57.02%) and BDAB maybe the ring opening of the terazine (theoretical value 32.72%). The left should be a certain product with high melt point.
The decomposition of explosives may be caused by the reactions in or between molecules at specific temperature. The decomposition of energetic salt composed ofcations and anions is possibly related to the nucleophilic reactions of anions or electrophilic reactions of cations.
To obtain the kinetic parameters [apparent activation energy, pre-exponential constant] and the most probable kinetic model functions of major exothermic decomposition reaction for DMAB, PDAB and BDAB, the five integral methods (General integral, MacCallum-Tanner, Šatava-Šesták, Agrawal, Flynn-Wall-Ozawa) and one differential method (Kissinger) [20, 24-25] are employed. The basic data for the main exothermic decomposition process used in calculated are listed in Table 3.
| Tab.3 The nonisothermal data of DMAB, PDAB and BDAB obtained by DSC curves at different heating rates |
The DSC curves at the heating rate of 5, 10, 15, 20 K·min-1 are dealt with mathematic means, and the temperature data corresponding to the conversion degrees (α) can be obtained. The values of Ea are obtained by Ozawa′s method from the isoconversional DSC curves at four different heating rates, and the relation Ea-α curves of three salts are displayed in Fig. 3. As can be seen from Fig. 3, the activation energy changes slightly in the section of 0.10-0.90(α) for DMAB, 0.20-0.80 for PDAB, and 0.10-0.50 for BDAB, so it is reliable to select the above mentioned sections to study the mechanism of the thermal decomposition reaction.
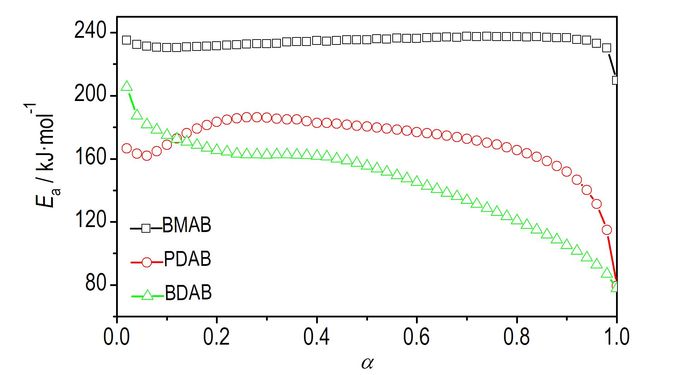
|
Fig.3 Ea/ α curves for the decomposition reaction of DMAB, PDAB and BDAB by Ozawa′s method |
Forty-one types of kinetic model functions and the basic data in Table 4 of DMAB, PDAB and BDAB are put into the integral and differential equations for calculation, the values of Ea, lg A, and linear correlation coefficient (r) are calculated with the linear least-squares method, and the most probable mechanism function is selected by the better value of r [26]. The results of satisfying the conditions at the same time are the final results as listed in Table 5, and the relevant function is the reaction mechanism function of the decomposition process.
| Tab.4 Thermal decomposition data determined by DSC curves at different heating rates for DMAB, PDAB and BDABK |
| Tab.5 Kinetic parameters for the main exothermic decomposition process of DMAB, PDAB and BDAB |
From Table 5, one can find that the values of Ea and lg A obtained from the nonisothermal DSC curves are in approximately good agreement with the values calculated by Kissinger′s method and Ozawa′s method, and the mechanism function number are determinated. Respectively substituting f(α) expression, the values of Ea/kJ·mol-1and A/s-1 into Eq. (1)
| $\text{d}\alpha /\text{d}t=Af(\alpha ){{\text{e}}^{-E/RT}}$ | (1) |
the following kinetic equations describing the main thermal decomposition process:
for DMAB,
d α=1018.02×5(1-α)[-ln(1-α)]3/5/2×exp(2.30×105/ RT)
for PDAB,
d α=1016.23×4 α3/4×exp(-1.85×105/ RT)
for BDAB,
d α=1016.58×5(1-α)[-ln(1-α)]3/5/2×exp(-1.93×105/ RT)
are obtained.
Because of the value of Ea for DMAB is the highest one, it can be deduced the thermal stability of DMAB is better than that of the others.
3.3 Thermal SafetyThe values (Te0 and Tp0) of the initial temperature point at which DSC curve deviates from the onset temperature (Te) and peak temperature (Tp) corresponding to β→0 are obtained by Eq.(2) (where b, c and d are coefficients), and the self-accelerating decomposition temperature (TSADT) is obtained by Eq.(3) [26-27]. The results are listed in Table 6.
| Tab.6 The thermal safty and thermodynamic properties of DMAB, PDAB and BDAB |
| ${{T}_{\text{e(or p}{{\text{)}}_{i}}}}={{T}_{\text{e0(or p0)}i}}+b{{\beta }_{i}}+c\beta _{i}^{2}+d\beta _{i}^{3}$ | (2) |
| ${{T}_{\text{SADT}}}={{T}_{\text{e0}}}~$ | (3) |
The thermal ignition temperature (Tbe0 or TTIT) is obtained by substituting EeO and Te0 into Zhang-Hu-Xie-Li equation Eq.(4) [26], and the critical temperatures of thermal explosion (Tp0 or Tb) is obtained by substituting EpO and Tp0 into the equation. The high value of Tb for DMAB (Table 6) illustrates the process of transition from thermal decomposition to thermal explosion is not easy to take palce.
| ${{T}_{\text{be0 (or bp0)}}}=~\frac{{{E}_{O}}-\sqrt{E_{O}^{2}-4{{E}_{O}}R{{T}_{\text{e0 (or p0)}}}}}{2R}$ | (4) |
The adiabatic time-to-explosion (tTIAD) of energetic materials (EMs) is the time of decomposition transiting to explosion under the adiabatic conditions, which is also an important parameter for assessing the thermal stability and safety.
Substituting the following data
for DMAB: cp=2.589-1.385×10-2 T+3.354 ×10-5 T2, E=246.02×103 J·mol-1, A=1019.35 s-1, Q=Δ H=1194.0 J·g-1, T= Tb=601.93 K, T0= Te0=576.87 K, mechanism function: f(α)=5(1-α)[-ln(1-α)]3/5/2
for PDAB: cp=-0.2813+5.365×10-3 T-2.993×10-7 T2, E=190.41×103 J·mol-1, A=1016.89 s-1, Q=Δ H=620.35 J·g-1, T= Tb=524.66 K, T0= Te0=511.90 K, mechanism function: f(α)=4 α3/4
for BDAB: cp=0.9018-2.297×10-3 T+1.239×10-5 T2, E=193.41×103 J·mol-1, A=1016.73 s-1, Q=Δ H=635.10 J·g-1, T= Tb=536.73 K, T0= Te0=521.55 K, mechanism function: f(α)=5(1-α)[-ln(1-α)]3/5/2
into Smith equation Eq.(5) and Eq.(6) [27-29]
| $\alpha = \frac{1}{{{Q_{\rm{d}}}A}}\int_{{T_0}}^T {\frac{{{c_p}}}{{{Q_{\rm{d}}}}}} {\rm{d}}T$ | (5) |
| ${{t}_{\text{TIAD}}}=\int_{0}^{t}{\text{d}t}=\frac{1}{{{Q}_{\text{d}}}A}\int_{{{T}_{0}}}^{T}{\frac{{{c}_{p}}\text{exp}~\left( E/RT \right)}{f\left( \alpha \right)}}\text{d}T$ | (6) |
the values of tTIAD for three salts are acquired and listed in Table 6.
It should be pointed out that the value of several parameters expect for Td (There are water molecular in DMAB, and H2O lost from the structure when the compound is heated from the room temperature) lines with the similar order, that is, BTATz>DMAB>BDAB>PDAB, which is in consistent with the result of Ea. Therefore, it can be predicted that the order of the thermal safety is as following: BTATz>DMAB>BDAB>PDAB.
Another valuable finding is the content of N has the affection to the thermal safety, and the thermal safety increases with the content of N growing.
3.4 Thermodynamic Properties of BTATz, DMAB, PDAB and BDABThe entropy of activation(Δ S≠), enthalpy of activation (Δ H≠), and free energy of activation (Δ G≠) of the main exothermic decomposition reaction, are obtained by Eqs.(7)-(9) with T= Tp0, E= EK, and A= A K[15, 25, 27-28], and also summarized in Table 6. The positive values of Δ G≠ indicates that the exothermic decomposition reaction can proceed under the heating condition.
| $A{\rm{exp}} - \frac{{{E_a}}}{{RT}} = \frac{{{k_B}T}}{h}{\rm{exp}} - \frac{{\Delta {G^ \ne }}}{{RT}}$ | (7) |
| $\Delta {H^ \ne } = {E_a} - RT$ | (8) |
| $\Delta {G^ \ne } = \Delta {H^ \ne } - T\Delta {S^ \ne }$ | (9) |
Where h is the plank constant(6.625×10-34J·s); kB is the Boltzmann constant(1.3807×10-23 J·K-1)
3.5 Detonation Velocity and Detonation Pressure of PDABThe detonation velocity (D) and detonation pressure (p) are the important targets of scaling the detonation characteristics of energetic materials, which can be predicted with the nitrogen equivalent equation (NE equation) shown as formulas (10)-(12) [30].
| $\sum N = \sum {x_i}{N_i}/M$ | (10) |
| $D = (690 + 1160{\rho _0})\sum N$ | (11) |
| $p = 1.092{({\rho _0}\sum N)^2} - 0.574$ | (12) |
Where ρ0 represents the density of an explosive, g·cm-3, ∑ N represents the total nitrogen equivalent of detonation product, Ni is the nitrogen equivalent value of the certain product, xi is the mole number of certain detonation product produced by a mole explosive.
According to the order of H2—CO—C in forming detonation products, the detonation products are determined as
| ${{\rm{C}}_7}{{\rm{H}}_{20}}{{\rm{N}}_{16}} = 10{{\rm{H}}_2} + 7{\rm{C}} + 8{{\rm{N}}_2}$ |
Through the nitrogen equivalent indexes of the detonation products in Table 7 with M=328, ρ0=1.504 g·cm-3, total nitrogen equivalents of PDAB are obtained
| Tab.7 Nitrogen equivalents of different detonation products |
| $\sum N = 100 \times \left( {10 \times 0.29 + 7 \times 0.15 + 8 \times 1} \right)/328 = 3.64$ |
Based on the Eqs.(11) and (12), the values of D and p for PDAB were obtained as 8862.09 m·s-1 and 32.15 GPa, respectively.
4 Conclusions(1) Three novel energetic salts of BTATz, DMAB, BDAB and PDAB, were synthesized and characterized. The Crystal structure of PDAB is monoclinic, space group C2/c. Moreover, many different hydrogen bonds existed in one unit of PDAB make the thermal stability of PDAB molecule increase.
(2) The density, decomposition temperature, detonation velocity and detonation pressure of PDAB are 1.504 g·cm-3, 530.05 K, 8862.09 m·s-1 and 32.15 GPa, respectively.
(3) Using TSADT as criterion, the heat-resisstance ability of three energetic salts of BTATz decreases in the order of DMAB(576.87 K)>BDAB(521.55 K)>PDAB(511.90 K).
(4) The thermal stability of BTATz, DMAB, PDAB and BDAB decreses in the order of BTATz>DMAB>BDAB>PDAB.
| [1] | Hickey M A, Chavez D E, Naud D. Preparation of 3, 3′-azobis(6-amino-1, 2, 4, 5-tetrazine): United States, US6342589[P]. 2002. |
| [2] | Hickey M A, Chavez D E, Naud D. 3, 6-bis(1 H-1, 2, 3, 4-tetrazol-5-ylamino)-1, 2, 4, 5-tetrazine or salt thereof: United States, US6657059[P]. 2003. |
| [3] | YUE Sou-ti, YANG Shi-qing. Synthesis and properties of 3, 6-bis(1 H-1, 2, 3, 4-tetrazol-5-yl-amino)-1, 2, 4, 5-tetrazine[J]. Chinese Journal of Energetic Material(Hanneng Cailiao), 2004, 12(3): 155-157. |
| [4] | WANG Bo-zhou, LAI Wei-peng, LIU Qian, et al. Synthesis, characterization and quantum chemistry study on 3, 6-bis(1 H-1, 2, 3, 4-tetrazol-5-yl-amino)-1, 2, 4, 5-tetrazine[J]. Chinese Journal of Organic Chemistry, 2008, 28(3): 422-427. |
| [5] | Saikia A, Sivabalan R, Polke B G, et al. Burn rate measurements of HMX, TATB, DHT, DAAF, and BTATz[J]. Journal of Hazardous Materials, 2009, 170(1): 306-311. DOI:10.1016/j.jhazmat.2009.04.095 |
| [6] | ZHANG Xing-gao, ZHU Hui, YANG Shi-qing, et al. Study on thermal decomposition kinetics and mechanism of nitrogen-rich compound BTATz[J]. Journal of Propulsion Technololgy, 2007, 28(3): 322-325. |
| [7] | Chavez D E, Hickey M A, Naud D. Tetrazine Exposives[J]. Propellants, Explosives, Pyrotechnics, 2004, 29(4): 209-215. DOI:10.1002/(ISSN)1521-4087 |
| [8] | Son S F, Berghout H L, Bolme C A. Burn rate measurements of HMX, TATB, DHT, DAAF, and BTATz[J]. Proceeding of the Combustion Institute, 2000, 28(1): 919-924. DOI:10.1016/S0082-0784(00)80298-2 |
| [9] | LI Na, ZHAO Feng-qi, LUO Yang, et al. Dissolution properties of 3, 6-bis(1 H-1, 2, 3, 4-tetrazol-5-yl-amino)-1, 2, 4, 5-tetrazine in N-methyl pyrrolidone and dimethyl sulfoxide[J]. Journal of Solution Chemistry, 2014, 43(7): 1250-1258. DOI:10.1007/s10953-014-0198-8 |
| [10] | ZHANG Xian-bo, REN Ying-hui, LI Wen, et al. A novel magnesium salt based on BTATz: crystal structure, thermal behavior and thermal safety[J]. Chemical Research in Chinese Universities, 2013, 29(4): 627-631. DOI:10.1007/s40242-013-2350-1 |
| [11] | ZHANG Xian-bo, REN Ying-hui, LI Wen, et al. 3, 6-Bis(1 H-1, 2, 3, 4-tetrazol-5-yl-amino)-1, 2, 4, 5-tetrazine-based energetic strontium (Ⅱ) complexes: synthesis, crystal structure and thermal properties[J]. Journal of Coordination Chemistry, 2013, 66: 2051-2064. DOI:10.1080/00958972.2013.796040 |
| [12] | LI Wen, REN Ying-hui, ZHAO Feng-qi, et al. Nitrogen-rich energetic zinc salt on BTATz: syntheses and thermodynamic[J]. Journal of Functional Materials, 2013, 44(22): 3326-3329. |
| [13] | LI Wen, REN Ying-hui, ZHAO Feng-qi, et al. Effects of lead complex-based BTATz on thermal behaviors, non-isothermal reaction kinetics and combustion properties of DB/RDX-CMDB propellants[J]. Acta Physico-Chimica Sinica, 2013, 29(10): 2087-2094. |
| [14] | REN Ying-hui, LI Wen, ZHANG Xian-bo, et al. Nonisothermal decomposition kinetics and thermal safety of Ag2(BTATz)·2H2O(BTATz=3, 6-bis(1 H-1, 2, 3, 4-tetrazole-5-amino)-1, 2, 4, 5-tetrazine)[J]. Journal of Applied Chemisty, 2013, 30(9): 1036-1041. |
| [15] | YI Jian-hua, ZHAO Feng-qi, WANG Bo-zhou, et al. Thermal behaviors, non-isothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant[J]. Journal of Hazardous Materials, 2010, 181: 432-439. DOI:10.1016/j.jhazmat.2010.05.029 |
| [16] | YI Jian-hua, ZHAO Feng-qi, WANG Bo-zhou, et al. BTATz-HNIW-CMDB propellants decomposition reaction kinetics and thermal safety[J]. Journal of Thermal Analysis and Calorimetry, 2014, 115(2): 1227-1234. DOI:10.1007/s10973-013-3400-0 |
| [17] | Hickey M A, Chavez D E, Naud D. Low-smoke pyrotechnic compositions: United States, US6312537[P]. 2001. |
| [18] | LI Shang-wen, ZHAO Feng-qi, YUAN Chao. Tendency of research and development for overseas solid propellants[J]. Journal of Solid Rocket Technology, 2002, 25(2): 36-42. |
| [19] | Hickey M A, Chavez D E, Naud D. Propellant containing 3, 6-bis(1 H-1, 2, 3, 4-tetrazol-5-yl-amino)-1, 2, 4, 5-tetrazine or salts thereof. US 6458227[P]. 2002. |
| [20] | Wang R H, Xu H Y, Guo Y, et al. Bis[3-(5-nitroimino-1, 2, 4-triazolate)]-based energetic salts: synthesis and promising properties of a new family of high-density insensitive materials[J]. Journal of the American Chemical Society, 2010, 132: 11904-11905. DOI:10.1021/ja1055033 |
| [21] | BI Fu-qiang, FAN Xue-zhong, Xu Cheng, et al. Review on insensitive non-metallic energetic ionic compounds of tetrazolate anions[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(6): 805-811. |
| [22] | ZHAI Lian-jie, FAN Xue-zhong, WANG Bo-zhou, et al. Synthesis and properties of 5-(3-amino-1, 2, 5-oxadiazol-4-yl) tetrazol-1-ol and its ammonium and hydroxylammonium salts[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2016, 24(9): 862-867. |
| [23] | LI Hui, YU Qian-qian, WANG Bo-zhou, et al. Synthesis and properties of 3, 3′-bis (tetrazol-5-yl)-4, 4′-azofurazan and its energetic salts[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2013, 21(6): 821-824. |
| [24] | Sheldric G M. SHELXL-97, Program for the refinement of crystal structure[CP], University of Gottingen, Germany 1997. |
| [25] | YI Jian-hua, ZHAO Feng-qi, GAO Hong-xu, et al. Preparation, characterization, nonisothermal reaction kinetics, thermodynamic properties, and safety performances of high nitrogen compound: hydrazine 3-nitro-1, 2, 4-triazol-5-one complex[J]. Journal of Hazardous Materials, 2008, 153: 261-268. DOI:10.1016/j.jhazmat.2007.08.064 |
| [26] | HU Rong-zu, GAO Sheng-li, ZHAO Feng-qi, et al. Thermal analysis kinetics (2th)[M]. 2008: 151-155. |
| [27] | ZHAOFeng-qi, HU Rong-zu, GAO Hong-xu, et al. New developments in hazardous materials research[M]. Nova Science Publishers Inc., New York, 2006: 161-167. |
| [28] | REN Ying-hui, ZHAO Feng-qi, YI Jian-hua, et al. Studies on an ionic compound (3-ATz)+ (NTO)-: crystal structure, specific heat capacity, thermal behaviors and thermal safety[J]. Journal of Iranian Chemical Society, 2012, 9: 407-414. DOI:10.1007/s13738-011-0051-6 |
| [29] | REN Ying-hui, LI Wen, ZHAO Feng-qi, et al. Crystal structure and thermal behaviors for 3, 5-dinitrobenzoic acid of 3, 5-diamino-1, 2, 4-triazole[J]. Journal of Analytical and Applied Pyrolysis, 2013, 102: 89-96. DOI:10.1016/j.jaap.2013.03.010 |
| [30] | CHEN Hua-xiong, CHEN Shu-sen, LI Li-jie, et al. Synthesis, single crystal structure and characterization of pentanitromonoformylhexaazaisowurtzitane[J]. Journal of Hazardous Materials, 2010, 175(1-3): 569-574. DOI:10.1016/j.jhazmat.2009.10.043 |

Three energetic salts based 3,6-bis(1H-1,2,3,4-tetrazol-5-yl-amino)-s-tetrazine(BTATz), DMAB, PDAB and PDAB were prepared and the crystal structure of 1,3-propanediamine salt was determined. The main thermal decomposition kinetic mechanisms of the three salts were obtained with non-isothermal method.




