Nowadays, many domestic enterprises show great interest in the zinc (Ⅱ) tri(carbohydrazide) perchlorate (GTX) of which is a new, highly-energetic and environmental friendly primary explosive. It is good enough to be applied in civil detonators. However, there are still some residues remaining in the mother liquor such as dissolved GTX, carbohydrazide(CHZ) and perchlorates according to the technical routine[1].
CHZ is a urea derivative, in which both the amide groups of urea are replaced by hydrazine(HZ). It can coordinate with many kinds of metal ions as a monodentate or multidentate[2-4] since it contains several function groups that possess lone electron pairs. Due to its strong reducibility, CHZ has also been used to design a variety of energetic coordination compounds such as cadmium tri(carbohydrazide) perchlorate[5] of which has been successfully prepared and proved to be remarkable in practical applications. These coordination compounds could act as primary explosives and components in propellants or high explosives[6-10].
GTX has been widely used in civil explosive enterprises now. And after a long period of GTX manufacture period, there is a large amount of CHZ remaining in its mother liquor of which is discharged as waste water. Since CHZ can react with the iron and perchloric acid very easily to form iron tri(carbohydrazide) perchlorate, an extremely sensitive compound that spontaneously explodes in solution. So it is necessary to remove or consume CHZ totally before discharging the waste water. In addition, CHZ is a dangerous grade chemical substance, with a dangerous level of 5-22-33-41-553[10]. The number 53 of 5-22-33-41-553 indicates a long-term detrimental effect on water environment. If the mass of toxic waste was poured outside, the natural living body would be harmed. In order to promote the application of GTX around the world widely, it is imperative to solve the problem of explosive waste water fundamentally during the GTX production processes. Thus, a practical and effective method of destroying CHZ should be developed.
Sodium percarbonate (SPC, 2Na2CO3·3H2O2, M=314.02) is an oxidant carbonate, which can react in solid state with reductants. The reaction exhibits complex multistep behaviors controlled by physic-geometrical and successive chemistry reaction schemes[12-14, 15-21]. The thermal decomposition of SPC can be empirically described as two overlapped reaction steps of endothermic and exothermic processes, which are separated by the physic-geometrical constraints and the changes in the self-generated reaction conditions. In addition, the thermal decomposition of SPC is considered as a successive process of the detachment and subsequent decomposition of H2O2(g)[22]. SPC is widely used in industries and laboratories as an effective oxidizing agent and a source of hydrogen peroxide. Due to stable, inexpensive, non-toxic, and easily handled characteristics, this chemical material is extensively used in detergent industry as a bleaching agent. Moreover, SPC has also been utilized in organic synthesis for oxidation reaction[23-24]. Herein, to explore the optimum parameters for the reaction between carbohydrazide and sodium percarbonate, classical chemical titration analysis was used to test the residue concentration of CHZ. A better technology for the reaction of CHZ and SPC was proposed by changing the volume ratio, concentration of SPC and the reaction time with the method of controlling variables.
2 Experimental 2.1 Materials and EquipmentCHZ, SPC, potassium iodate, trichloromethane, hydrochloric acid, transfer pipette (25.00 mL), measuring cylinder (50 mL 5 mL) and acid burette (25.00 mL) were commercially obtained from Beijing Tong Guang Fine Chemical Company. All the solvents and reagents were analytical grade and used without further purification.
2.2 Experiment Procedure 2.2.1 Reactant RatioThe titration principle of the mixed solutions with potassium iodate is carried out according to the redox equation (1):
| $ {\rm{2KI}}{{\rm{O}}_{\rm{3}}}{\rm{ + }}{{\rm{N}}_{\rm{4}}}{{\rm{H}}_{\rm{6}}}{\rm{CO + 4HCl}} \to {\rm{2KCl + 2 I Cl + 2}}{{\rm{N}}_{\rm{2}}}{\rm{ + C}}{{\rm{O}}_{\rm{2}}}{\rm{ + 5}}{{\rm{H}}_{\rm{2}}}{\rm{O}} $ | (1) |
The Jamieson iodate method for the quantitative determination of hydrazine and its salts has also been successfully applied to the estimation of primary hydrazino groups in some compounds, such as hydrazide[25].
9.0080 g (0.1 mol) of CHZ was dissolved in 250 mL deionized water in a beaker and transferred into a 1 L volumetric flask. The beaker and glass bar were rinsed for three times with deionized water. Then, deionized water was added into the volumetric flask to the scale mark. Similarly, 7.8505 g (0.025 mol) of SPC was disposed to obtain 0.1 mol·L-1 SPC solution.
There were five steps in the first experiment. Firstly, 50 mL of 0.1 mol·L-1 CHZ solution was added into 300 milliliter beaker. Then, 50 mL of 0.1 mol·L-1 SPC solution was added. The next, four glasses of 50 mL CHZ solutions were mixed with 100 mL 0.1 mol·L-1 SPC, 150 mL 0.1 mol·L-1 SPC, 200 mL 0.1 mol·L-1 SPC and 250 mL 0.1 mol·L-1 SPC, respectively.
All of the five solutions were stirred at 80 ℃ for 30 min. After the reaction, 100 mL of each reacted solutions were taken into five 250 mL volumetric flasks, and the deionized water was added to the scale mark. The solutions were calibrated to the scale with deionized water, respectively. So the solutions were all diluted 2.5 times. At last, the solutions were tested according to the titration process.
The titration process: 25.00 mL sample solution was transferred into a 250 mL conical flask with a 25.00 mL pipette. Then, 50.00 mL of 10% (mass percent) hydrochloric acid solution was added. After the temperature of the solution was close to room temperature, the solution was titrated with 0.05 mol·L-1 potassium iodate standard solution until the solution color changed from brown to light yellow. Then, 5.00 mL of trichloromethane was added into the titration reaction solution again, which was then titrated slowly with heavily shaking until the orange color of the chloroform layer disappeared. All the samples were titrated repeatedly three times in the same manner.
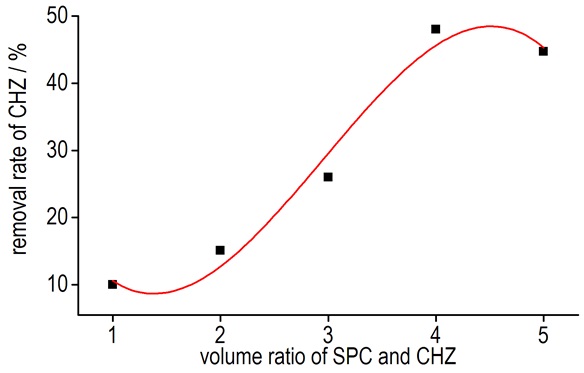
The average consumption of potassium iodate and the reduction ratio was obtained and listed in Table 1. The change curve for the removal rate of CHZ with the volume ratio of CHZ and SPC was shown in Fig. 1.
| Tab.1 Effects of different reaction material ratio on the consumption of potassium iodate and the removal rate of CHZ |
|
Fig.1 The change curve for the removal rate of CHZ to the volume ratio of SPC and CHZ |
Results indicated that 50 mL of 0.1 mol·L-1 CHZ solution mixed with 200 mL of 0.1 mol·L-1 of SPC solution possessed the highest removal rate of CHZ (48%). Therefore, the reaction was proved to be very effective when the reactant volume was 50 mL (mol·L-1) CHZ to 200 mL (mol·L-1) SPC. So the solution volume ratio was determined as 1:4.
2.2 The Concentration of SPC SolutionFrom the procedure 2.2.1, the optimum reactant ratio was obtained as 50 mL of CHZ solution with 200 mL of SPC solution. Thus, the effect of SPC concentrations on the reaction was carried out in this experiment. After controlling the fixed concentration (0.1 mol·L-1) of CHZ, SPC concentration was changed in order to explore the best concentration of SPC.
8.6356, 9.4206, 10.2057, 10.9907 g and 11.7758 g of SPC were dissolved into five beakers, respectively. These solutions were transferred into five 250 mL volumetric flasks, and enough deionized water was poured into the flasks to the scale mark respectively. So 0.11 mol·L-1, 0.12 mol·L-1, 0.13 mol·L-1, 0.14 mol·L-1, 0.15 mol·L-1 SPC solutions were well prepared.
There were also five steps in the experiment.
Firstly, 50 mL of 0.1 mol·L-1 CHZ solution was added into a 300 milliliter beaker, 200 mL of 0.11 mol·L-1 SPC solution was added. In the same way, five glasses of 50 mL 0.1 mol·L-1 CHZ solution were mixed with 200 mL 0.12, 0.13, 0.14 mol·L-1 and 0.15 mol·L-1 SPC solutions respectively.
All of the five mixtures were stirred at 80 ℃ for 30 min. Then, all of the five solutions were taken into five 250 mL volumetric flasks respectively.
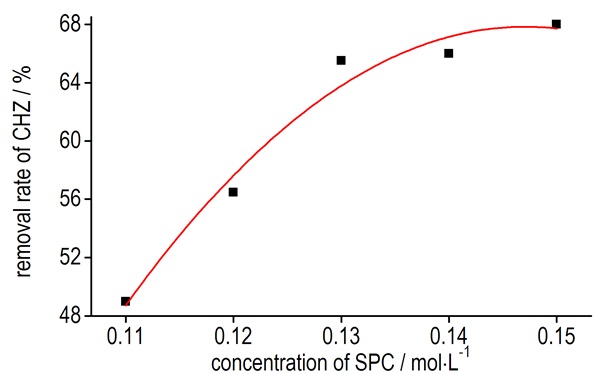
The five solutions were titrated in the same way as the same as procedure 2.2.1. The consumption of potassium iodate solution and the reduction ratio were obtained and listed in Table 2. The change curve for the removal rate of CHZ with the different concentration of SPC was listed in Fig. 2.
| Tab.2 Effects of different SPC concentrations on the consumption of potassium iodate and the removal rate of CHZ |
|
Fig.2 The change curve for the removal rate of CHZ to the different concentration of SPC |
The results showed that 50 mL of 0.1 mol·L-1 CHZ solution mixed with 200 mL of 0.15 mol·L-1 SPC solution possessed the highest removal rate of 68%. Therefore, results indicated that the removal rate of CHZ increases with the rise of SPC's concentration (from 0.11 mol·L-1 to 0.15 mol·L-1) when the volume ratio (1:4) was curtained. The best concentration of SPC should be controlled in 0.15 mol·L-1.
2.3 Reaction TimeFrom the procedure 2.2.1 and 2.2.2, a better volume ratio of CHZ solution and SPC solution was obtained as 1:4. The removal rate increases with the increase of SPC's concentration (from 0.11 mol·L-1 to 0.15 mol·L-1). The effects of reaction time on the CHZ consumption was explored at the optimized reaction conditions.
Eight glasses of 50 mL of 0.1 mol·L-1 CHZ solutions were mixed with eight 200 mL of 0.15 mol·L-1 SPC solutions. The mixtures were stirred at 80 ℃ for 20, 30, 40, 50, 60, 90, 120, 150 min respectively.
After the reaction, all of the solutions were taken into five 250 mL volumetric flasks, respectively.
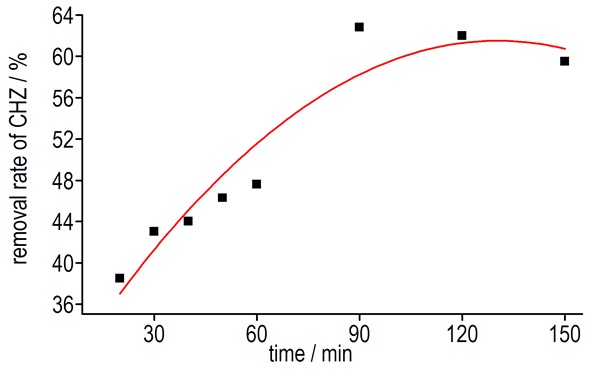
All of the solutions were titrated according to the procedure 2.2.1. The consumption of potassium iodate and the reduction ratio were obtained and listed in Table 3. The change curve for the removal rate of CHZ with different time was listed in Fig. 3.
| Tab.3 Effects of different reaction time on the consumption of potassium iodate and the removal rate of CHZ |
|
Fig.3 The change curve for the removal rate of CHZ with different time |
Results showed that when the volume ratio (1:4) and the concentration (0.1 mol·L-1 CHZ and 0.15 mol·L-1 SPC) were curtained, the removal rate of CHZ increased with the increasing of reaction time. The best reaction duration should be no less than 90 min.
3 Results and DiscussionsAll the titration operations were carried out in triplicate. And the results were calculated based on data from blank experiments. The data of all the experiments are real and exact, which indicated a better volume ratio is 1:4, a better concentration of SPC is 0.15 mol·L-1, and the best reaction time is at least 90 min.
Kinetics studies can be carried out according to the concentration of the residue in CHZ solution. Linear regression method was used to determine the best-fitting kinetic models.
The first order kinetics rate equations can be written in integral the equation as[26-28]:
| $ \frac{{{\rm{d}}{q_t}}}{{{\rm{d}}t}} = {k_1}\left( {{q_e} - {q_t}} \right) $ | (2) |
Where, t is reaction time, min; qe is the amount of reaction concentration of CHZ at equilibrium, mg·g-1; qt is the amount of reaction concentration of CHZ at any time, mg·g-1; k1 stands for the first order rate constant, min-1.
When: t=0, qt=0; which has a linear form of:
| $ {\rm{ln}}\left( {{q_e} - {q_t}} \right) = {\rm{ln}}\;{q_e} - {k_1}t $ | (3) |
The second order kinetic model can be written in integral the equation as[29-30]:
| $ \frac{{{\rm{d}}{q_t}}}{{{\rm{d}}t}} = {k_2}{\left( {{q_e} - {q_t}} \right)^2} $ | (4) |
Where, k2 stands for the second order rate constant, g·(mg·min)-1,
When: t=0, qt=0;which has a linear form of:
| $ \frac{t}{{{q_t}}} = \frac{1}{{{k_2}q_e^2}} + \frac{1}{{{q_e}}}t $ | (5) |
The first order rate constants can be determined experimentally by plotting ln(qe-qt) against t. Similarly, the second order rate constants can be determined by plotting t/qt against t.
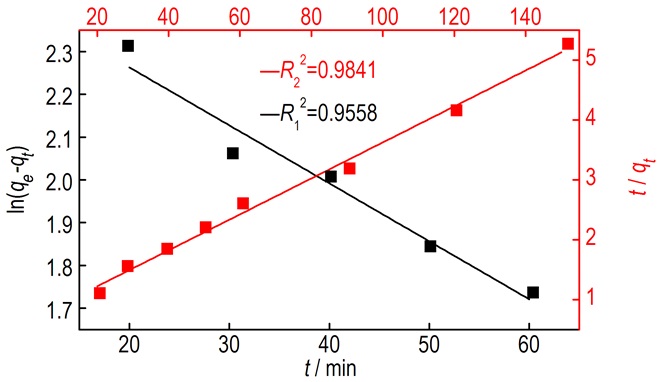
According to the linear least-squares method, the first order and the second order kinetic equations were summarized and the parameters obtained by treating CHZ data were listed in Table 4, (Δm represents the amount of CHZ on reaction, mg, ) which are the parameters of first and second order kinetics model.
| Tab.4 Kinetic parameters obtained by treating CHZ data |
|
Fig.4 The first and second order kinetic model analysis curves for the reaction of CHZ with SPC in solution |
The linear coefficient R2 of the first order was 0.9558, and the linear coefficient R2 of the second order was 0.9841. Obviously, fitting all the experimental data with the second order kinetic model was better.
4 Conclusions(1) It is the first time to obtain the optimal reaction conditions of CHZ and SPC and the chemical kinetics equation and its curves. All of these make significant contributions to the removal of CHZ in the GTX solutions.
(2) When the solution volume ratio of CHZ (0.1 mol·L-1) and SPC (0.1 mol·L-1) was kept at the range from 1 to 4, the reaction proved to be very sufficient.
(3) When the reactant ratio (1:4) was curtained, the removal rate of CHZ increased with the increase of the SPC's concentration in solution (from 0.11 mol·L-1 to 0.15 mol·L-1). And the best concentration is 0.15 mol·L-1.
(4) The removal rate of CHZ increased with the delay of mixing reaction time. And the optimum time is at least 90 min. The-second-order linear kinetics model reaches the highest linear fitting degree for the reaction of CHZ with SPC.
| [1] |
ZHANG Zhi-gang, ZHANG Jian-guo, ZHANG Tong-lai. Preparation technique and explosive properties of[Zn(CHZ)3](ClO4)2[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2001, 9(2): 49-52. |
| [2] |
Akiyoshi M, Nakamura H, Hara Y. The thermal behavior of the zinc complexes as a non-azide gas generant for safer riving-Zn complexes of the carbohydrazide and semicarbazide[J]. Propellants, Explosives, Pyrotechnics, 2000, 25(1): 41-46. DOI:10.1002/(ISSN)1521-4087 |
| [3] |
Madaan A, Kikuchi S, Bhalla S. The thermal behavior of the carbohydrazide complexes of certain metals (3) The gas evolved in the decomposition of the Mg complex[J]. Journal of Japan Explosives Society, 1997, 58(2): 68-75. |
| [4] |
Talawar M B, Agrawal A P, Chhabra J S, et al. Energetic co-ordination compounds: synthesis, characterization and thermolysis studies on bis-(5-nitro-2H-tetrazolato-N2) tetraammine cobalt (Ⅲ) perchlorate (BNCP) and its new transition metal (Ni/Cu/Zn) perchlorate analogues[J]. Journal of Hazardous Materials, 2005, 120(1-3): 25-35. DOI:10.1016/j.jhazmat.2004.12.021 |
| [5] |
ZHANG Tong-lai, WEI Zhao-rong. Research on the primary explosive GTG[J]. Journal of Hazardous Materials, 1999, 28: 16-19. |
| [6] |
ZHANG Jian-guo, ZHANG Tong-lai, WEI Zhao-rong. Studies on preparation, crystal structure and application of[Mn(CHZ)3](ClO4)2[J]. Chemical Journal of Chinese Universities-Chinese, 2001, 22(6): 895-897. |
| [7] |
ZHANG Tong-Lai, LU Chun-hua, ZHANG Jian-guo, et al. Preparation and molecular structure of {[Ca(CHZ)2(H2O)](NTO)2·3.5H2O}n[J]. Propellants, Explosives, Pyrotechnics, 2003, 28(5): 271-276. DOI:10.1002/(ISSN)1521-4087 |
| [8] |
SUN Yuan-hua, ZHANG Tong-lai, ZHANG Jian-guo, et al. Flash pyrolysis study of zinc carbohydrazide perchlorate using T-jump/FTIR spectroscopy[J]. Combustion Flame, 2006, 145(3): 643-646. DOI:10.1016/j.combustflame.2006.01.014 |
| [9] |
SUN Yuan-hua, ZHANG Tong-lai, ZHANG Jian-guo, et al. Decomposition kinetics of manganese tris(carbohydrazide) perchlorate (MnCP) derived from the filament control voltage of the T-jump/FTIR spectroscopy[J]. International Journal of Thermal Sciences, 2006, 45(8): 814-818. DOI:10.1016/j.ijthermalsci.2005.10.008 |
| [10] |
ER Shi-yong, SONG Wen-hua, BAI Ru. A brief analysis of classification of hazardous chemicals[J]. Safety and environmental engineering, 2006, 13(4): 35-38. |
| [11] |
Sinditskii V P, Fogelizang A E, Dutov M D, et al. Structure of complex compounds of metal chlorides, sulfates, nitrates and perchlorates with carbohydrazide[J]. Inorganic Chemistry, 1987, 32(8): 1944-1949. |
| [12] |
Wada T, Koga N. Kinetics and mechanism of the thermal decomposition of sodium percarbonate: role of the surface product layer[J]. Journal of Physical Chemistry A, 2013, 117: 1880-1889. |
| [13] |
Wada T, Nakano M, Koga N. Multistep kinetic behavior of the thermal decomposition of granular sodium percarbonate: hindrance effect of the outer surface layer[J]. Journal of Physical Chemistry A, 2015, 119(38): 9749-60. DOI:10.1021/acs.jpca.5b07042 |
| [14] |
Nagaishi T, Yoshimura J, Matsumoto M, et al. Thermal decomposition of the addition compound of sodium carbonate with hydrogen peroxide (Na2CO3·1.5H2O2)[J]. Journal of Thermal Analysis and Calorimetry, 1980, 18(3): 501-508. DOI:10.1007/BF01910231 |
| [15] |
Galwey A K, Hood W J. Thermal decomposition of sodium carbonate perhydrate in the solid state[J]. Chemischer Informationsdienst, 1979, 10(40): 1810-1815. |
| [16] |
Galwey A K, Hood W J. Thermal decomposition of sodium carbonate perhydrate in the presence of liquid water[J]. Journal of the Chemical Society, Faraday Transactions, 1982, 13(52): 391-396. |
| [17] |
Lunghi A, Cattaneo M, Cardillo P. Thermal stability of sodium percarbonate: kinetic data[J]. Rivista dei Combustibili, 1996, 50(6): 229-234. |
| [18] |
QIN Fu-yuan. Thermal decomposition reaction kinetics of sodium percarbonate[J]. East China University of Science & Technology, 2001, 27: 214-216. |
| [19] |
WANG Hong-xian, ZHAO Hong-kun, DU Mei-Ju, et al. Research on thermal decomposition kinetics of sodium[J]. Shangqiu Teachers College, 2003, 19: 94-97. |
| [20] |
WANG Hong-xian, ZHAO Hong-kun. Theoretical analysis of the thermal decomposition kinetics of sodium percarbonate and its experimental verification[J]. Nantong University(Natural Science Edition), 2006, 5: 43-46. |
| [21] |
Gigante L, Dellavedova M, Pasturenzi C, et al. Study of the decomposition mechanism of sodium carbonate perhydrate by thermal analysis[J]. La rivista dei combustibili e dell'Industia Chimica, 2008, 62: 279-284. |
| [22] |
Nagaishi T, Kaneda T, Kotsubo A, et al. Thermal decomposition of the addition compound of sodium carbonate with hydrogen peroxide (Na2CO3·1.5H2O2)[J]. Kogyo Kayaku, 1976, 37: 84-90. |
| [23] |
Kabalka G W, Wadgaonkar P P, Shoup T M. Sodium percarbonate: a convenient reagent for efficiently oxidizing organoboranes[J]. Tetrahedron Letters, 1989, 30(38): 5103-5104. DOI:10.1016/S0040-4039(01)93458-6 |
| [24] |
Kabalka G W, Wadgaonkar P P, Shoup T M. Oxidation of organoboranes with sodium percarbonate[J]. Organometallics, 2002, 9(4): 1316-1320. |
| [25] |
Keim G I, Henry R A, Smith G B L. The oxidation of di-and triaminoguanidine with potassium iodate[J]. Journal of the American Chemical Society, 1950, 72(11): 4944-4946. DOI:10.1021/ja01167a029 |
| [26] |
Sen T K, Sarzali M V. Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminum oxide (Al2O3): a kinetic and equilibrium study[J]. Chemical Engineering Journal, 2008, 142(3): 256-262. DOI:10.1016/j.cej.2007.12.001 |
| [27] |
Bhattacharya A K, Naiya T K, Mandal S N, et al. Adsorption, kinetics and equilibrium studies on removal of Cr(Ⅵ) from aqueous solutions using different low-cost adsorbents[J]. Chemical Engineering Journal, 2008, 137(3): 529-541. |
| [28] |
Tseng R L, Wu F C, Juang R S. Liquid-phase adsorption of dyes and phenols using pinewood-based activated carbons[J]. Carbon, 2003, 41(3): 487-495. DOI:10.1016/S0008-6223(02)00367-6 |
| [29] |
Ho Y S. Review of second-order models for adsorption systems[J]. Journal of Hazardous Materials, 2006, 136(3): 681-9. DOI:10.1016/j.jhazmat.2005.12.043 |
| [30] |
Azizian S. Kinetic models of sorption: a theoretical analysis[J]. Journal of Colloid and Interface Science, 2004, 276(1): 47-52. DOI:10.1016/j.jcis.2004.03.048 |

The chemical kinetics and process optimization for the reaction of carbohydrazide with sodium percarbonate were fully studied.




