2. State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi′an 710065, China
2. 氟氮化工资源高效开发与利用国家重点实验室, 陕西 西安 710065
N-oxides have been extensively studied in the field of energetic materials[1-2]. Recently, the benzotriazol-3-ium-1-oxide compounds have attracted more attentions owing to their low sensitivities towards shock, friction, heat and electrostatic discharge[3]. For example, 4, 6-dinitrobenzotriazol-3-ium-oxide (DNBTO) was identified as a kind of potential explosive with the high-performance and insensitivity. It has some desirable traits, including a low impact sensitivity (20 J), and a low friction sensitivity (>360 N)[4]. In order to search for the novel energetic derivatives with higher energy than DNBTO, a novel compound, 4, 6-dinitrobenzotriazol-3-dinitromethyl-1-oxide (TNBTO) was designed and synthesized from DNBTO. Comparing with DNBTO, TNBTO exhibits higher density and detonation velocity because of the introduce of a dinitromethyl group[5-9]. In view of the above observations, the detailed studies of the synthesis and characterization of TNBTO were carried out in this work. In addition, the detonation parameters and stability were investigated.
2 Experimental 2.1 Materials and Instruments4, 6-Dinitrobenzotriazol-3-ium-1-oxide (DNBTO) was prepared and purified according to the reference[10], and other reagents were purchased from the commercial sources. 1H NMR and 13C NMR were obtained in DMSO-d6 on a Bruker AV500 NMR spectrometer. Infrared spectra were obtained from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Elemental analyses (C, H and N) were performed on a VARI-El-3 elemental analyzer.
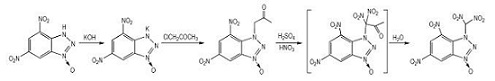
2.2 Synthesis and CharacterizationUsing 4, 6-dinitrobenzotriazol-3-ium-1-oxide (DNBTO) as starting materials, the title compound TNBTO was firstly synthesized via the reactions of metathesis, substitution and nitration-hydrolysis (Scheme 1).
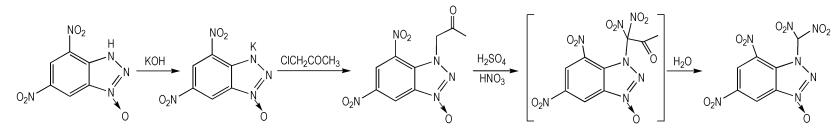
|
Scheme1 |
DNBTO (1.0 g, 4.44 mmol) was dissolved in 80 mL ethanol, and then potassium hydroxide (0.25 g, 4.46 mmol) dissolved in a minimal amount of water was added dropwise at 40 ℃. The solution was stirred at 40 ℃ for other 2 h. After evaporation of the solvent, the residue was washed with diethyl ether, and dried to give 1.0 g purple solid with a yield of 85.5%. IR (KBr, ν/cm-1):3106, 2398, 1765, 1643, 1559, 1508, 1438, 1384, 1341, 1189, 1157, 1055, 983, 933, 885, 826, 806. 1H NMR (DMSO-d6, 500 MHz), δ: 9.096 (1H, CH), 8.880(1H, CH); 13C NMR (DMSO-d6, 125 MHz), δ: 144.830, 137.430, 136.783, 130.260, 117.535, 115.295; Anal.Calcd. for C6H2 N5O5K (%): C 27.38, H 0.77, N 26.61; Found: C 27.41, H 0.83, N 26.32.
2.2.2 4, 6-Dinitrobenzotriazol-3-Acetone-1-OxidePotassium bromide (0.5 g, 4.2 mmol) and potassium 4, 6-dinitrobenzotriazol-3-ium-1-oxide (0.95 g, 3.6 mmol) were dissolved in 80 mL acetone at ambient temperature. To the reaction mixture, chloroacetone (0.38 g, 4 mmol) was added dropwise. The solution was stirred for 8 h at 58 ℃. After evaporation of the solvent, the residue was washed with water and diethyl ether, and dried to give 0.41 g orange solid with a yield of 40.5% and a purity of 99.2%(HPLC). IR (KBr, ν/cm-1): 3444, 3097, 2986, 2938, 2869, 1745, 1632, 1601, 4534, 1488, 1401, 1371, 1344, 1281, 1234, 1184, 1169, 1101, 1067, 1020, 998, 935, 911, 805; 1H NMR (DMSO-d6, 500 MHz), δ: 9.370(1H, CH), 8.938(1H, CH), 5.603 (2H, CH2), 2.147(3H, CH3); 13C NMR (DMSO-d6, 125 MHz), δ: 202.223, 146.033, 137.988, 137.217, 117.602, 115.371, 84.192, 26.524; Anal.Calcd. for C9H7 N5O6(%): C 38.44, H 2.51, N 24.91; Found: C 38.40, H 2.75, N 24.82.
2.2.3 4, 6-Dinitrobenzotriazol-3-Dinitromethyl-1-Oxide (TNBTO)4, 6-Dinitrobenzotriazol-3-acetone-1-oxide was dissolved in 5 mL 98% sulfuric acid. To the reaction mixture, 65% nitric acid (3.9 mL, 54 mmol) was added dropwise at-5 ℃. The solution was stirred for 6 h at 40 ℃. Then the reaction mixture was poured into ice water. The yellow precipitate was filtered to obtain 1.0 g solid with a yield of 52.6%. IR (KBr, ν/cm-1): 3422, 3105, 2289, 1603, 1543, 1489, 1346, 1296, 1233, 1174, 1121, 1065, 1000, 934, 910, 847, 804, 776; 1H NMR (DMSO-d6, 500 MHz), δ: 9.52-9.52 (1H, CH), 9.02-9.343(1H, CH), 7.23-7.03(H, (NO2)2); 13C NMR (DMSO-d6, 125 MHz), δ: 146.61, 143.53, 133.31, 130.31, 123.16, 118.80, 76.95; Anal.Calcd. for C7H3N7O9(%): C 25.54, H 0.92, N 29.79; Found: C 25.50, H 1.04, N 29.33.
3 Physicochemical and Energetic PropertiesAll the quantum computations were performed using the Gaussian 09 (Revision A. 02) suite of programs[11]. The optimized structures were characterized to be true local energy minima on the potential-energy surface without imaginary frequencies. The densities of DNBTO and TNBTO were computed based on Monte-Caolo method using the optimized structure at the B3LYP/6-311+G (d, p) level of theory [12-13]. The gas phase heats of formation were calculated by the atomization method using the Gaussian 09 program package at the CBS-4M level of theory [14]. Gas phase heat of formation was transformed to solid phase heat of formation by Trouton′s rule [15]. Based on the calculated density and heat of formation, the detonation velocity and detonation pressure for DNBTO and TNBTO were calculated by Kamlet-Jacobs equations [16]. The stability was analyzed by TLC. The properties of TNBTO were obtained by calculation or test as follows: density is 1.81 g·cm-3, detonation velocity is 8161.2 m·s-1, heat of formation is 143.7 kJ·kg-1. Due to the introduce of R—C (NO2)2 group, TNBTO exhibits a higher density and detonation velocity compared with DNBTO.However, the heat of formation of TNBTO was lower than that of DNBTO, and TNBTO showed a relatively poor stability because it easily decomposes at room temperature. The physicochemical and detonation properties of DNBTO and TNBTO were listed in Table 1.
| Tab.1 The performances of DNBTO and TNBTO |
(1) TNBTO was firstly synthesized using 4, 6-dinitrobenzotriazol-3-ium-1-oxide (DNBTO) as raw material via the reactions of metathesis, substitution and nitration-hydrolysis. Its structure was characterized by IR, NMR and element analysis.
(2) The main performance of TNBTO were obtained by theoretical calculation as follows: density is 1.81 g·cm-3, detonation velocity is 8161.2 m·s-1, heat of formation is 143.7 kJ·kg-1.
(3) TNBTO was easily decomposed at room temperature, and showed a relatively poor thermal stability.
| [1] | Šarlauskas J, AnuseviCius Ž, Misiūnas A. Benzofuroxan (benzo[1, 2-c]1, 2, 5-oxadiazole N-oxide) derivatives as potential energetic materials: studies on their synthesis and properties[J]. Central European Journal of Energetic Materials, 2012(9): 365-386. |
| [2] | Dippold A A, Klapötke T M. A Study of dinitro-bis-1, 2, 4-triazole-1, 1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides[J]. Journal of the American Chemical Society, 2013, 135: 9931-9938. DOI:10.1021/ja404164j |
| [3] | Kehler J, Püschl A, Dahl O. Improved synthesis of 1-hydroxy-4-nitro-6-trifluoromethylbenzotriazole and 1-hydroxy-4, 6-dinitrobenzotriazole[J]. Acta Chemica Scandinavica, 1996, 50: 1171-1173. DOI:10.3891/acta.chem.scand.50-1171 |
| [4] | Klapötke T M, Pflüger C, SuCeska M. Zwitterionic explosives based on 4, 6-dinitrobenzotriazol-3-ium-1-oxide[M]. New Trends Res. Energ. Mater, Proc. Semin. 17th, Pardubice, Czech: 2014: 754-768. |
| [5] | Semenov VV, Kanischev M I, Shevelev S A, et al. Thermal ring opening reaction of N-polynitromethyl tetrazoles: facile generation of nitrilimines and their reactivity[J]. Tetrahedron, 2009, 65: 3441-3445. DOI:10.1016/j.tet.2009.02.032 |
| [6] | Katritzky A R, Sommen G L, Gromova A V, et al. Synthetic routes towards tetrazolium and triazolium dinitromethylides[J]. Chemistry of Heterocyclic Compounds, 2005, 41(1): 111-118. DOI:10.1007/s10593-005-0116-5 |
| [7] | Astrat′ev A A, Dashko D V, Stepanov A I. Reactivity of 2-(dinitromethylene)-4, 6-dihydroxy-5, 5-dinitropyrimidine in the processes of nucleophilic subatitution[C] //ICT, 2005: 444-450. |
| [8] | Klapotke T M, Xaver F S. Dinitromethyltetrazole and its salts-A comprehensive study[J]. Propellants, Explosives, Pyrotechnics, 2010, 35: 114-129. DOI:10.1002/prep.200900049 |
| [9] | ZHANG Min, GE Zhong-xue, BI Fu-qiang, et al. Synthesis and performance of 2-dinitromethyl-5-nitrotetrazole[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao), 2013, 21(5): 688-690. |
| [10] | HUO Huan, WANG Bozhou, ZHAI Lian-jie, et al. An Insensitive energetic compound 5, 7-diamino-4, 6-dinitro-benzotriazol-3-ium-1-oxide: synthesis, characterization and performances[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao), 2016, 24(9): 857-861. |
| [11] | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, rev. A. 02[CP], Gaussian, Inc. , Wallingford, CT, 2009. |
| [12] | Qiu L, Xiao H M, Gong X D, et al. Crystal density predictions for nitramines base on quantum chemistry[J]. Journal of Hazardous Materials, 2007, 141(1): 280-288. DOI:10.1016/j.jhazmat.2006.06.135 |
| [13] | Rice B M, Hare J J, Byrd E F. Accurate predictions of crystal densities using quantum mechanical molecular volumes[J]. The Journal of Physical Chemistry A, 2007, 111(42): 10874-10879. DOI:10.1021/jp073117j |
| [14] | Montgomery J A, Frisch M J, Ochterski J W, et al. A complete basis set model chemistry Ⅶ. use of the minimum population localization method[J]. The Journal of Physical Chemistry, 2000, 112: 6532-6542. DOI:10.1063/1.481224 |
| [15] | Westwell M S, Searle M S, Wales D J, et al. Empirical correlation between thermodynamic properties and intermolecular forces[J]. Journal of the American Chemical Society, 1995, 117: 5013-5015. DOI:10.1021/ja00123a001 |
| [16] | Kamlet M J, Jacobs S J. Chemistry of detonation Ⅰ. A simple method for calculating detonation properties of CHNO explosives[J]. The Journal of Physical Chemistry, 1968, 48(1): 23-35. DOI:10.1063/1.1667908 |
The effects of desorption time and foaming temperature on the combustion performance of microcellular oblate spherical propellants with layered structure were investigated.




