2. China Research and Development Academy of Machinery Equipment, Beijing 100076, China
2. ${affiVo.addressStrCn}
Energetic organic nitrogen-rich compounds have currently attracted significant attention from many researchers because of their novel properties, for example, high density, high positive heat of formation, and thermal stability[1-8]. Most of the compounds are mainly based on nitrogen-rich heterocycles, such as triazine[1-2], triazole[3-5], tetrazine[6-7], tetrazole[8]. Recently, as a fairly new class of energetic nitrogen-rich heterocycles, benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide has gained increasing attention because it has not only high density but also good oxygen balance[9]. In particularly, this heterocycle show remarkably high stability, with many members of this highly energetic class of compounds decomposing above 200 ℃[9-10].
Up to date, many synthetic strategies have developed for the synthesis of benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide[11-16]. But, these methods suffer from extremely harsh reaction condition, poor yield and troublesome purification processes. For example, oxidation of corresponding anilines with Caro′s acid gives nitroso derivative in rather low yield along with intractable side product because nitroso compound is very unstable and readily overoxidized. Particularly, the key intermediate 2-amino-1-(tert-butyl-NNO-azoxy)benzene has been previously performed through coupling chloro derivative with liquid ammonia, but high pressure (253.25×105 Pa), high temperature (190 ℃) and anhydrous solvent were necessary. These procedures do not seem to be operationally simple or safe (Scheme 1)[12]. Hence, developing new way to obtain benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide would be very fascinating. Here we report a mild and convenient procedure for the synthesis of benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide.

|
Scheme1 Previous method for the synthesis of benzo-1, 2, 3, 4-tetrazine 1, 3-dioxide[12] |
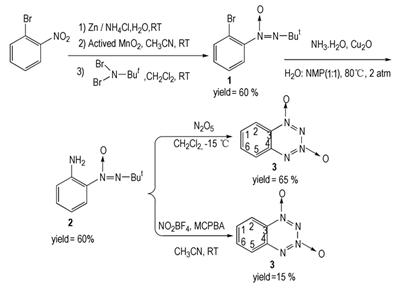
The sequence of reaction steps in total synthesis of benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide is shown in Scheme 2. 2-Bromonitrobenzene was firstly reduced with zinc dust and ammonium chloride at room temperature to obtain 2-bromo-1-hydroxylaminobenzene in 70% yield. Next, oxidation of the hydroxylamino derivative with actived manganese oxide gave 2-bromo-1-nitrosobenzene in 20% yield. 2-Bromo-1-nitrosobenzene was treated with N,N-dibromo-tert-butylamine according to the Kovacic method to give 2-bromo-1-(tert-butyl-NNO-azoxy)benzene (1) in 90% yield. In attempt to improve the obtained yield, the tandem reaction was also developed to successfully obtain compound 1 in 60% overall yield. Then, compound 1 was reacted with aqueous ammonia using Cu2O as the catalyst at 80 ℃ to give 2-amino-1-(tert-butyl-NNO-azoxy)benzene (2) in 60% yield. Here, a mild copper-catalyzed amination reaction was utilized to avoid harsh reaction condition. Finally, the benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide was obtained by the cyclization reaction between compound 2 with N2O5 in 65% yield while the previous method gave a lower yield of 6%[11]. Moreover, N2O5 is commercially unavailable and have to be prepared with complicated, dangerous processes[17]. To avoid the employment of N2O5, the same transformation in the study was also performed using readily available NO2BF4 (96%) and MCPBA (m-chloroperbenzoic acid) and obtained benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide in 15% yield. The structure of target compound was confirmed by1H NMR, 13C NMR and by the mass spectrometry (EI-MS). Compound 3 is yellow solid, m.p. 168 ℃. 1H NMR (400 MHz, d6-DMSO): δ 8.31 (d, 1H, J=8.8 Hz), 8.16 (t, J=15.2 Hz, 1H), 7.98 (d, J=8.4 Hz, 1H), 7.88 (t, J=15.6 Hz, 1H); 13C NMR (100 MHz, d6-DMSO): δ 143.9, 138.9, 132.3, 128.7, 124.8, 119.4; MS (EI): m/z=165 [M+1]+, 164 [M]+, 136, 120, 108, 91, 76, 64; IR (KBr, cm-1): 3093, 1585, 1500, 1470, 1414, 1352, 1281, 1174, 948, 775, 667, 561; Anal. Calcd for C10H15N3O: C 43.91; H 2.46; N 34.14. Found: C 43.87, H 2.73, N 34.31. In the 1H NMR spectra, the compound possesses four protons. The H-2 and H-5 appear as two doubles at 7.98 (J=8.4 Hz) and 8.31 (J=8.8 Hz), respectively. The two triplets at 7.88 (J=15.6 Hz) and 8.16 (J=15.2 Hz) assign to H-6 and H-1. In the 13C NMR spectra, the benzene ring of benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide yields six separate peaks. The assignments for these carbon atoms are 143.9 : C4, 138.9: C6, 132.3: C1, 128.7: C3, 124.8: C5, 119.4: C2. Mass spectral data for compound 5 shows M+1 peak at 165, which is in good agreement with its molecular weight of M+1 species.

|
Scheme2 A convenient synthesis of benzo-1, 2, 3, 4-tetrazine-1, 3-dioxide |
In summary, we devised a convenient procedure for the synthesis of benzo-1, 2, 3, 4-tetrazine 1, 3-dioxide from simple raw material. In particular, the use of Cu2O as catalyst made the amination reaction simple, safe and efficient. Its ease of work-up, fairly mild reaction conditions provides a new access to benzo-1, 2, 3, 4-tetrazine 1, 3-dioxide.
| [1] |
Huynh M H V, Hiskey M A, Hartline E L. Polyazido high-nitrogen compounds: hydrazo and azo-1, 3, 5-triazine[J]. Angew Chem Int Ed, 2004, 43(37): 4924-4928. DOI:10.1002/(ISSN)1521-3773 |
| [2] |
LI Y C, ZHANG X J, ZHAO C L, et al. Synthesis, characterization and thermal decomposition mechanism of 4, 4′, 6, 6′-tetra(azido)azo-1, 3, 5-triazine(TAAT)[J]. Chin Org Chem,, 2011, 31: 1484-1489. |
| [3] |
LI Y C, QI C, LI S H, et al. 1, 1′-Azobis-1, 2, 3-triazole: A high-nitrogen compound with stable N8 structure and photochromism[J]. J Am Chem Soc,, 2010, 132(35): 12172-12173. DOI:10.1021/ja103525v |
| [4] |
Qi C, Li S H, Pang S P, et al. A novel stable high-nitrogen energetic material: 4, 4′-azobis(1, 2, 4-triazole)[J]. J Mater Chem,, 2011, 21: 3221-3225. DOI:10.1039/c0jm02970j |
| [5] |
LI S H, PANG S P, LI X T, et al. Synthesis of new tetrazene(N-N=N-N)-linked bi(1, 2, 4-triazole)[J]. Chin Chem Lett,, 2007, 18: 1176-1178. DOI:10.1016/j.cclet.2007.08.018 |
| [6] |
Huynh M H V, Hiskey M A, Chavez D E, et al. Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen C—N compound[J]. J Am Chem Soc,, 2005, 127(36): 12537-12543. DOI:10.1021/ja0509735 |
| [7] |
Li X T, Pang S P, Yu Y Z, et al. Synthesis and theoretical studies of 3, 6-diazido-1, 2, 4, 5-tetrazine[J]. Acta Chim Sinica,, 2007, 65(10): 971-976. |
| [8] |
Göbel M, Karaghiosoff K, Klapötke T M, et al. Nitrotetrazolate-2N-oxides and the strategy of N-oxide introduction[J]. J Am Chem Soc,, 2010, 132(48): 17216-17226. DOI:10.1021/ja106892a |
| [9] |
Churakov A M, Tartakovsky V A. Progress in 1, 2, 3, 4-tetrazine chemistry[J]. Chem Rev,, 2004, 104(5): 2601-2616. DOI:10.1021/cr020094q |
| [10] |
Schechter H, Venugopal M, Srinivasulu D. Synthesis of 1, 2, 3, 4-tetrazines, 1, 2, 3, 4-tetrazine di-N-oxides, pentazole derivatives, pentazine poly N-oxides, and nitroacetylenes[R]. Ohio State University. Research Foundations, DARPA/AFOSR Sponsored, Project 746566. March 8, 2006.
|
| [11] |
Churakov A M, Smirnov O Y, Loffe S L, et al. Benzo-1, 2, 3, 4-tetrazine 1, 3-dioxides: synthesis and NMR study[J]. Eur J Org Chem,, 2002, 2002(14): 2342-2349. DOI:10.1002/1099-0690(200207)2002:14<2342::AID-EJOC2342>3.0.CO;2-H |
| [12] |
Frumkin A E, Churokov A M, Strelenko Y A, et al. Synthesis of amino-substituted 1, 3-bis(tert-butyl-NNO-azoxy) benzenes 1, 4-amino and 4, 6-diamino dervatives[J]. Russian Chemical Bulletin,, 1999, 48(7): 1295-1298. DOI:10.1007/BF02495293 |
| [13] |
Smirnov O Y, Churakov A M, Tyurin A Y, et al. Nucleophilic substitution in benzo-1, 2, 3, 4-tetrazine-1, 3-dioxides[J]. Russian Chemical bulletin, International Edition,, 2002, 51(10): 1849-1856. DOI:10.1023/A:1021396317258 |
| [14] |
Churakov A M, Loffe S L, Tartakovskii V A. The first synthesis of 1, 2, 3, 4-tetrazine-1, 3-di-N-oxides[J]. Mendeleev Commun, 1991, 1(3): 101-103. DOI:10.1070/MC1991v001n03ABEH000062 |
| [15] |
Klenov M S, Zelenov V P, Churakov A M, et al. Generation of oxodiazonium ions: synthesis of benzotetrazine-1, 3-dioxides from 2-(tert-butyl-NNO-azoxy)-N-nitroanilines[J]. Russian Chemical bulletin, International Edition,, 2011, 60(10): 2040-2045. DOI:10.1007/s11172-011-0310-9 |
| [16] |
Frumkin A E, Churakov A M, Strelenko Y A, et al. New approach to the synthesis of benzo[e][1, 2, 3, 4]tetrazine 1, 3-dioxides[J]. Russian Chemical Bulletin, 2000, 49(3): 480-484. |
| [17] |
Caesar G V, Goldfrank M. Nitration of starches with nitrogen pentoxide in presence of sodium fluoride[J]. J Am Chem Soc,, 1946, 68(3): 372-375. DOI:10.1021/ja01207a007 |