2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China
2. 西安近代化学研究所, 陕西 西安 710065
1, 1-Diamino-2, 2-dinitroethylene (FOX-7) is a novel high-energy material with high thermal stability, low sensitivity to impact and friction, and have been studied a lot[1-8]. FOX-7 is a representative "push-pull" nitro-enamine, and possesses a highly polarized carbon-carbon double bond with positive and negative charges being stabilized by the amino group and nitro group respectively, and presents certain acidic properties [9-11]. Its energetic salts, such as potassium salt, rubidium salt, cesium salt and guanidine salt, have been reported[11-13]. Other salts and metal complexes of FOX-7 also are synthesized throng replacement reaction[14-17]. Cu(NH3)2(FOX-7)2 is a new typical FOX-7 complex, and its synthesis and crystal structure have been reported[14-15] recently. In this paper, non-isothermal decomposition kinetics, specific heat capacity and adiabatic time-to-explosion of Cu(NH3)2(FOX-7)2 will be studied to further evaluate its thermal stability.
2 Experimental 2.1 SampleCu(NH3)2(FOX-7)2 was prepared according to Ref.[14]. Cu(NH3)2(FOX-7)2 is purple. Crystal data of Cu(NH3)2(FOX-7)2: monoclinic, space group P21/c, a=0.68818(7) nm, b=0.73083(8) nm, c=1.31966(14) nm, β=95.986°, V=0.66009(12) nm3, Dc=1.972 g·cm-3, μ=0.862 mm-1, F(000)=199, Z=2, R1=0.0344, wR2=0.0989. The molecular structure of Cu(NH3)2(FOX-7)2 is shown in Figure 1.
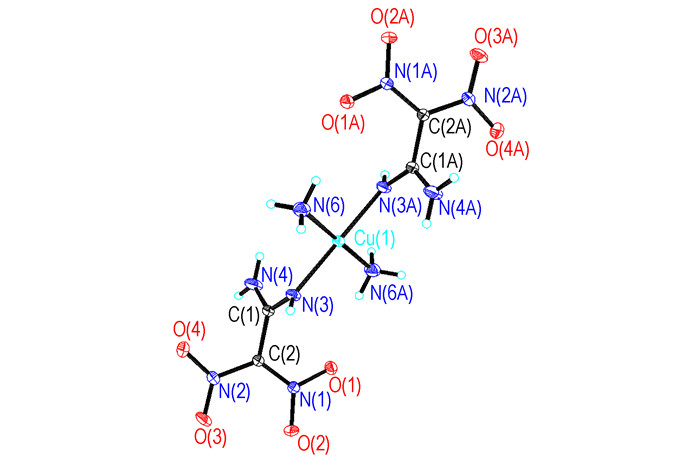
|
Fig.1 Molecular structure of Cu(NH3)2(FOX-7)2 |
The DSC experiments were performed using a DSC200 F3 apparatus (NETZSCH, Germany) under a nitrogen atmosphere at a flow rate of 80 mL·min-1. The sample was about 0.4 mg. The heating rates were 5.0, 7.5, 10.0, 12.5 ℃·min-1, respectively. The temperature range was from room temperature to 300 ℃.
The TG/DTG experiment was performed using a SDT-Q600 apparatus (TA, USA) under a nitrogen atmosphere at a flow rate of 100 mL·min-1. The sample was 0.32 mg. The heating rate was 5 ℃·min-1 and the temperature range was from room temperature to 350 ℃.
The specific heat capacity was determined using a Micro-DSCⅢ apparatus (SETARAM, France). The sample was 383.62 mg; heating rate was 0.15 ℃·min-1; temperature range was from 10 ℃ to 80 ℃.
3 Results and discussion 3.1 Thermal decomposition behaviorTypical DSC and TG-DTG curves of Cu(NH3)2(FOX-7)2 sample at a heating rate of 5.0 ℃·min-1 are shown in Figure 2 and Figure 3.
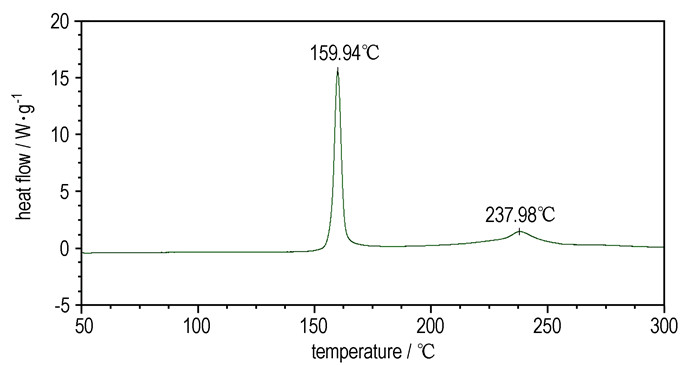
|
Fig.2 DSC curve of Cu(NH3)2(FOX-7)2 at 5.0 ℃·min-1 |
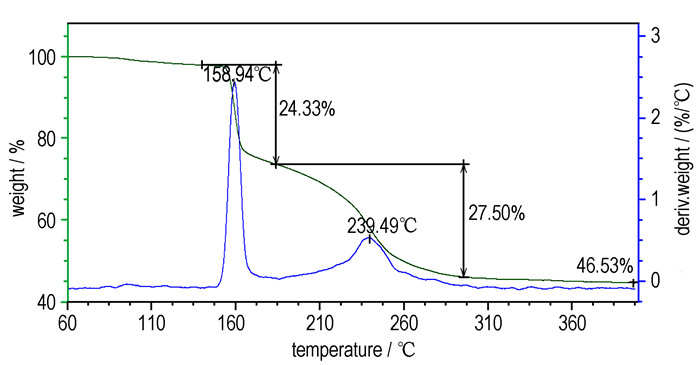
|
Fig.3 TG/DTG curves of Cu(NH3)2(FOX-7)2 at 5.0 ℃·min-1 |
From Figure 2 and Figure 3, the thermal decomposition of Cu(NH3)2(FOX-7)2 can be divided into two exothermic processes. The first is an intensive exothermic decomposition process, which occurs at 160~185 ℃ with a mass loss of about 24.3%. The extrapolated onset temperature, peak temperature and decomposition heat of Cu(NH3)2(FOX-7)2 are 157.2, 159.9 ℃ and 758.4 J·g-1, respectively. The second is a slow exothermic decomposition process with a mass loss of about 27.5 % at the temperature range of 185~300 ℃, and the peak temperature is 238.0 ℃. The final residue at 400 ℃ is about 46.5 %.
3.2 Non-isothermal decomposition kineticsIn order to obtain the kinetic parameters (the apparent activation energy (E) and pre-exponential constant (A)) of the first exothermic decomposition process, a multiple heating method (Kissinger method [18] and Ozawa method [19]) was employed. The determined values of the beginning temperature (T0), extrapolated onset temperature (Te) and peak temperature (Tp) at the different heating rates are listed in Table 1.The values of T00 and Te0 corresponding to β→0 obtained by Eq. (1) are also listed in Table 1[20].
| $ {T_{{\rm{0i}}\;{\rm{or}}\;{\rm{ei}}}} = {T_{{\rm{00}}\;{\rm{or}}\;{\rm{e0}}}} + n{\beta _{\rm{i}}} + m\beta _i^2,\;\;\;i = 1 \sim 4 $ | (1) |
| Tab.1 T0, Te, Tp, T00, Te0 and kinetic parameters of Cu(NH3)2(FOX-7)2 at various heating rates |
where n and m are coefficients.
The calculated values of kinetic parameters (E and A) are also listed in Table 1. The apparent activation energy obtained by Kissinger method is consistent with that obtained by Ozawa method. The linear correlation coefficients (rk, ro) are all close to 1.
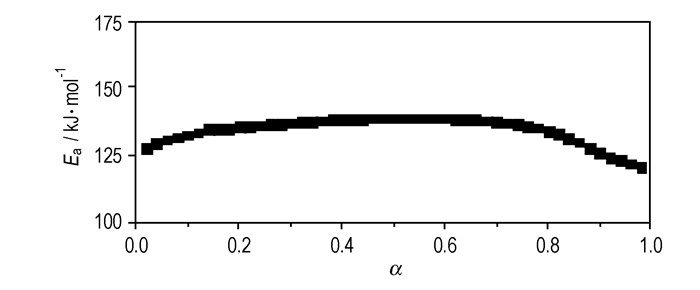
T versus α (the conversion degree) curves at different heating rates are shown in Figure 4. The values of E for any given value of α were obtained and shown in Figure 5. The values of E steadily distribute from 129 kJ·mol-1 to 139 kJ·mol-1 in the α range of 0.05~0.95, and the average value of E is 136.2 kJ·mol-1, which is in approximate agreement with that obtained by Kissinger method and Ozawa method from only peak-temperature values. So, the values were used to check the validity of E by other methods.
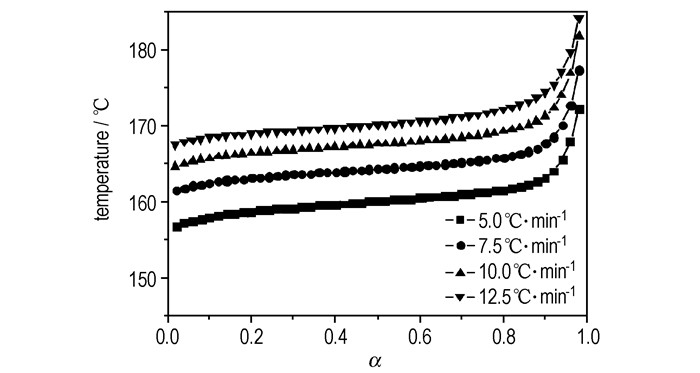
|
Fig.4 T vs α curves for the decomposition reaction of Cu(NH3)2(FOX-7)2 at different heating rates |
|
Fig.5 Ea vs α curve for the decomposition reaction of Cu(NH3)2(FOX-7)2 by Ozawa method |
The integral equations (The general integral equation, universal integral equation, MacCallum-Tanner equation, Šatava-Šesták equation and Agrawal equation) were cited to obtain the values of E, A and the most probable kinetic model function [f(α)] from each DSC curve. Forty-one types of kinetic model functions taken from Ref.[20] and experimental data form each DSC curve were put into the above five integral equationsfor calculating, respectively. So, the most probable kinetic model function is classified as f(α)=4α3/4(Mampel power law, n=1/4), according to the unanimity rule of calculation results from each model equation[20]. The kinetic equation can be described as:
| $ \frac{{{\rm{d}}\alpha }}{{{\rm{d}}T}} = \frac{{{{10}^{15.12}}}}{\beta }4{\alpha ^{3/4}}\exp ( - 1.429 \times {10^5}/\mathit{RT}) $ | (2) |
The self-accelerating decomposition temperature (TSADT) and critical temperature of thermal explosion (Tb) are two important parameters for energetic materials, which are required to ensure safe storage and process operations and then to evaluate the thermal stability. TSADT and Tb can be obtained by Eq.(3) and Eq.(4)[20-21], respectively.
| $ {T_{{\rm{SADT}}}} = {T_{e0}} $ | (3) |
| $ {T_{\rm{b}}} = \frac{{{E_{\rm{O}}} - \sqrt {E_{\rm{O}}^{\rm{2}} - 4{E_{\rm{O}}}R{T_{{\rm{e0}}}}} }}{{2R}} $ | (4) |
TSADT and Tb for Cu(NH3)2(FOX-7)2 are 145.5 ℃ and 156.2 ℃ respectively, which are much lower than those of FOX-7(206.0 ℃ and 207.1 ℃[3]).
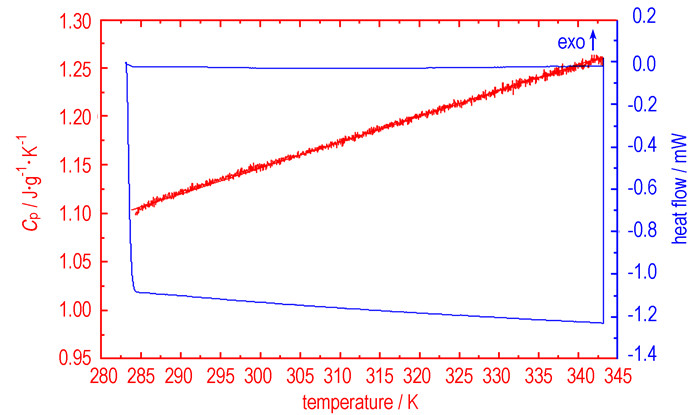
3.4 Specific heat capacityFig. 6 shows the determination result of Cu(NH3)2(FOX-7)2, using a continuous specific heat capacity mode of Micro-DSCⅢ. In determined temperature range, specific heat capacity presents a good linear relationship with temperature. Specific heat capacity equation of Cu(NH3)2(FOX-7)2 is:
| $ {C_{\rm{p}}} = 0.3468 + 2.6667 \times {10^{ - 3}}T(283.0K < \mathit{T} <343.0K) $ | (5) |
|
Fig.6 Determination results of the continuous specific heat capacity of Cu(NH3)2(FOX-7)2 |
The molar heat capacity of Cu(NH3)2(FOX-7)2 is 447.3 J·mol-1·K-1 at 25 ℃.
3.5 Adiabatic Time-to-explosionThe adiabatic time-to-explosion is also an important parameter for evaluating the thermal stability of energetic materials and can be calculated by Eq.(6) and Eq.(7) [20, 22-25].
| $ {C_{\rm{p}}} = \frac{{dT}}{{dt}} = QA\exp ( - E/RT)f(\alpha ) $ | (6) |
| $ \alpha = \int_{{T_0}}^T {\frac{{{C_{\rm{p}}}}}{Q}} {\rm{d}}T $ | (7) |
After integrating of Eq. (6), the adiabatic time-to-explosion equation can be obtained as:
| $ t = \int_0^t {{\rm{d}}t = } \int_{{T_0}}^T {\frac{{{C_{\rm{p}}}\exp (\mathit{E}/\mathit{RT})}}{{QAf(\alpha )}}} {\rm{d}}T $ | (8) |
where the limit of temperature integration is from T00 to Tb.
The conversion degree (α) of energetic materials from the beginning thermal decomposition to thermal explosion in the adiabatic conditions is too small (0.007) to obtain the most probable kinetic model function[f(α)] in the process. So, we used Reaction-order model [f(α)=(1-α)n] to estimate the adiabatic time-to-explosion and supposed different rate orders (n=0, 1, 2) [20, 26]. t0=9.45 s, t1=9.52 s and t2=9.59 s from Eq.(8) were obtained directly according to the above data. As a rule, the rate order (n) of energetic materials ranges from 0 to 2, therefore the adiabatic time-to-explosion of Cu(NH3)2(FOX-7)2 is about 9.5 s. Although it is a short time, the result can be proved credible according to the intense change of DSC curves in the first exothermic decomposition process.
4 Conclusions(1) The thermal decomposition of Cu(NH3)2(FOX-7)2 presents two continuous exothermic processes. The kinetic equation of the first decomposition process is
(2) The self-accelerating decomposition temperature and critical temperature of thermal explosion are 145.5 and 156.2 ℃, respectively. Specific heat capacity equation of Cu(NH3)2(FOX-7)2 is Cp= 0.3468+2.6667×10-3 T (283.0 K < T < 343.0 K) and the molar heat capacity is 447.3 J·mol-1·K-1 at 25 ℃. Adiabatic time-to-explosion of Cu(NH3)2(FOX-7)2 was estimated to be about 9.5 s.
(3) Results show that the thermal stability of Cu(NH3)2(FOX-7)2 is much lower than that of FOX-7.
| [1] |
Latypov N V, Bergman J, Langlet A, et al. Synthesis and reaction of 1, 1-diamino-2, 2-dinitroethylene[J]. Tetrahedron, 1998, 54: 11525-11536. DOI:10.1016/S0040-4020(98)00673-5 |
| [2] |
Bemm U, Östmark H. 1, 1-Diamino-2, 2-dinitroethylene: a novel energetic material with infinite layers in two dimensions[J]. Acta Crystallographica Section C, 1998, 54: 1997-1999. |
| [3] |
Gao H X, Zhao F Q, Hu R Z, et al. Thermochemical properties, thermal behavior and decomposition mechanism of 1, 1-diamino-2, 2dinitroethylene (DADE)[J]. Chinese Journal of Chemistry, 2006, 24: 177-181. DOI:10.1002/(ISSN)1614-7065 |
| [4] |
Majano G, Mintova S, Bein T, et al. Confined detection of high-energy-density materials[J]. The Journal of Physical Chemistry, 2007, 111(18): 6694-6699. |
| [5] |
Trzciński W A, Cudzilo S, Chylek Z, et al. Detonation properties of 1, 1-diamino-2, 2-dinitroethene (DADNE)[J]. Journal of Hazardous Materials, 2008, 157: 605-612. DOI:10.1016/j.jhazmat.2008.01.026 |
| [6] |
Zhao J J, Liu H. High-pressure behavior of crystalline FOX-7 by density functional theory calculations[J]. Computational Materials Science, 2008, 42: 698-703. DOI:10.1016/j.commatsci.2007.10.008 |
| [7] |
Huang B, Qiao Z Q, Nie F D, et al. Fabrication of FOX-7 quasi-three-dimensional grids of one-dimensional nanostructures via a spray freeze-drying technique and size-dependence of thermal properties[J]. Journal of Hazardous Materials, 2010, 184: 561-566. DOI:10.1016/j.jhazmat.2010.08.072 |
| [8] |
Venkatesan V, Polke B G, Sikder A K. Ab initio study on the intermolecular interactions between 1, 1-diamino-2, 2-dinitroethylene and acetylene: Pull effect on complex formation[J]. Computer Theoretical Chemistry, 2012, 995: 49-54. DOI:10.1016/j.comptc.2012.06.028 |
| [9] |
Rajappa S. Nitroenamines preparation, structure and synthetic potential[J]. Tetrahedron, 1981, 37: 1453-1480. DOI:10.1016/S0040-4020(01)92085-X |
| [10] |
Herve G, Guy J, Latypov N. The reactivity of 1, 1-diamino-2, 2-dinitroethene (FOX-7)[J]. Tetrahedron, 2005, 61: 6743-6748. DOI:10.1016/j.tet.2005.05.010 |
| [11] |
Herve G, Jacob G, Latypov. Novel illustrations of the specific reactivity of 1, 1-diamino-2, 2-dinitroethene (DADNE) leading to new unexpected compounds[J]. Tetrahedron, 2007, 63: 953-959. DOI:10.1016/j.tet.2006.11.031 |
| [12] |
X u, K Z, Chang, C R, Song J R, et al. Preparation, crystal structure and theoretical calculation of G(FOX-7)[J]. Chinese Journal of Chemistry, 2008, 26: 495-499. DOI:10.1002/(ISSN)1614-7065 |
| [13] |
Luo J A, Xu K Z, Wang M, et al. Syntheses and thermal behaviors of Rb(FOX-7)·H2O and Cs(FOX-7)·H2O[J]. Bulletin of the Korean Chemical Society, 2010, 31(10): 2867-2871. DOI:10.5012/bkcs.2010.31.10.2867 |
| [14] |
CHEN Yong-Shun, XU Kang-Zhen, WANG Min, et al. Crystal structure and thermal behavior of Cu(NH3)2(FOX-7)2[J]. Chinese Journal of Explosives & Propellants, 2011, 34(4): 5-9. |
| [15] |
Garg S, Gao HaiXiang, Parrish D A, et al. FOX-7(1, 1-Diamino-2, 2-dinitroethene): trapped by copper and amines[J]. Inorganic Chemistry, 2011, 50: 390-395. DOI:10.1021/ic102136r |
| [16] |
Vo T T, Parrish D A, Shreeve J M. 1, 1-Diamino-2, 2-dintroethene (FOX-7) in copper and nickel diamine complexes and copper FOX-7[J]. Inorganic Chemistry, 2012, 51: 1963-1968. DOI:10.1021/ic202288t |
| [17] |
He F, Xu K. Z, Zhang H, et al. Two new copper-FOX-7 complexes: synthesis, crystal structure, and thermal behavior[J]. Journal of Coordination Chemistry, 2013, 66(5): 845-855. DOI:10.1080/00958972.2013.771182 |
| [18] |
Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29: 1702-1706. DOI:10.1021/ac60131a045 |
| [19] |
Ozawa T. A method of analying thermogravimetric data[J]. Bulltin of Chemical Society Jpn, 1965, 38: 1881-1886. DOI:10.1246/bcsj.38.1881 |
| [20] |
Hu R Z, Gao S L, Zhao F Q, et al. Thermal Analysis Kinetics (2th)[M]. Beijing: Science Press, Beijing, 2008.
|
| [21] |
Zhang T L, Hu R Z, Xie Y, et al. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC[J]. Thermochimica Acta, 1994, 244: 171-176. DOI:10.1016/0040-6031(94)80216-5 |
| [22] |
Xu K Z, Song J R, Zhao F Q, et al. Thermal behavior, specific heat capacity and adiabatic time-to-explosion of G(FOX-7)[J]. Journal of Hazardous Materials, 2008, 158: 333-339. DOI:10.1016/j.jhazmat.2008.01.077 |
| [23] |
Ma H X, Yan B, Li Z N, et al. Preparation, non-isothermal decomposition kinetics, heat capacity and adiabatic time-to-explosion of NTO·DNAZ[J]. Journal of Hazardous Materials, 2009, 169: 1068-1073. DOI:10.1016/j.jhazmat.2009.04.057 |
| [24] |
Xu K Z, Chen Y S, Wang M, et al. Synthesis and thermal behavior of 4, 5-dihydroxyl-2-(dinitromethylene)-imidazolidine (DDNI)[J]. Journal Thermal Analysis and Calorimetry, 2011, 105: 293-300. DOI:10.1007/s10973-010-1244-4 |
| [25] |
Smith L C. An approximate solution of the adiabatic explosion problem[J]. Thermochimica. Acta, 1975, 13(1): 1-6. DOI:10.1016/0040-6031(75)80060-8 |
| [26] |
Vyzovkin S, Burnham A K, Criado J M, et al. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochimica Acta, 2011, 520: 1-19. DOI:10.1016/j.tca.2011.03.034 |
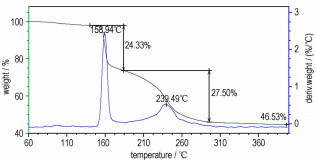
The thermal decomposition of Cu(NH3)2(FOX-7)2 presents two continuous exothermic processes. The kinetic equation of the first decomposition process is




