New high-energy density materials (HEDMs) not only need to enhance detonation pressure and detonation velocity, but also consider heat of formation, density and thermal stability[1-2]. Energy-rich functional groups including nitro (—NO2), nitrato (—ONO2), nitramine (—NNO2) and azido (—N3) as substituents into energetic compounds are an effective way to obtain better detonation performances[3]. Energetic compounds that contain nitramine (—NNO2) moiety are the most attractive and attainable at present, which is due to the enhanced nitrogen content, density and oxygen balance resulting from the presence of nitramine group[4-7]. Over the past decades, many nitramine-based explosives have been developed and used widely, including 1, 3, 5-trinitro-1, 3, 5-triazinane (RDX), 1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocane (HMX), 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazatetracyclo[5.5.0.0.0]dodecane (CL-20) and 1, 3, 3-trinitroazetidine (TNAZ), etc[8-9]. 1, 3-Dichloro-2-nitro-2-azapropane (DCNP) is a useful precursor that can be regarded as a promising compound to design and synthesize energetic nitramine ones. Some energetic compounds, such as 1, 7-diamino-1, 7-dinitrimino-2, 4, 6-trinitro-2, 4, 6-triazaheptane (APX), bis(2-(5-nitrotetrazol-2-yl)-2, 2-dinitroethyl)nitramine (BNTDNEA) and bis((5-nitro-2H-tetrazol-2-yl)methyl)nitramine (BNTMNA), have been synthesized by our group[10-11]. In this work, as part of our continual effort in nitramine energetic compounds, we reported the synthesis of N, N-bis((3, 5-dinitro-1H-1, 2, 4-triazol-1-yl)methyl)nitramine (BDNTMN) by N-alkylation reaction with potassium salt of 3, 5-dinitro-1, 2, 4-triazole (DNTK) and 1, 3-dichloro-2-nitro-2-azapropane (DCNP) and studied its thermal behavior and detonation performance.
2 Experimental 2.1 General Methods and MaterialsMelting points were determined using an open capillary tube. Infrared (IR) spectra were recorded on a Nicolet NEXUS 870 Infrared spectrometer using KBr pellets. 1H NMR and 13C NMR spectra were recorded on a Bruker AV 500 NMR spectrometer with TMS as the internal standard. Mass spectra were acquired using a GCMS-QP 2010 Micromass UK spectrometer. Elemental analyses were performed on a VARI-EI-3 elemental analyzer.
3, 5-Diamino-1, 2, 4-triazole (DAT) and DCNP were self-synthesized. Sodium nitrite, sulfuric acid, aminosulfuric acid, magnesium sulfate, ether, ethanol, potassium hydroxide and N, N′-dimethyl formamide were AR grade, purchased from Chengdu Kelong Chemical Reagents Factory.
2.2 Synthetic RouteBDNTMN was synthesized using DAT and DCNP as starting materials. The synthetic route was as follow (Scheme 1).
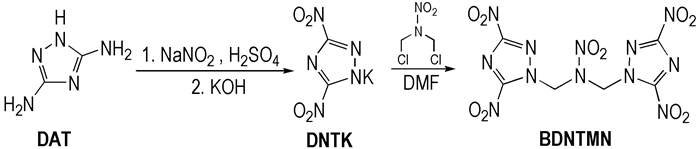
|
Scheme1 Synthetic route of BDNTMN |
To a solution of 54.2 g (0.786 mol) of sodium nitrite in 63.3 mL water at -10--5℃, a solution of 8.0 g (0.081 mol) of DAT in 169.5 mL 1.2 mol·L-1 sulfuric acid was added for 1.5 h, the reaction mixture was heated up to 60 ℃ and stirred for 1 h, then cooled the reaction mixture to room temperature. 26.9 mL 6.0 mol·L-1 sulfuric acid and 4.7 g (0.048 mol) aminosulfuric acid were added to reaction system and stirred for another 0.5 h, extracted with 90 mL×5 of ether, dried with magnesium sulfate, and the solvent was removed under reduced pressure, and 7.89 g yellow oil 3, 5-dinitro-1, 2, 4-triazole (DNT) was obtained with a yield of 61.4%. 1H NMR (DMSO-d6, 500 MHz) δ: 6.424 (s, 1H, NH); 13C NMR (DMSO-d6, 125 MHz) δ: 162.56; IR (KBr, ν/cm-1): 3224 (—NH—), 1659, 1382 (—NO2); MS (m/z): 159[M+]; Anal. calcd for C2H3N5O5: C 13.57, N 39.55, H 1.71; found C 13.73, N 38.91, H 2.13.
2.0 g (0.13 mol) of DNT was dissolved in 15.0 mL ethanol, then 14.2 g 5% potassium hydroxide (0.13 mol) was added dropwise at 5-10 ℃. After 2 h at room temperature, the precipitate was filtered off, washed with ethanol and ice-water, and dried to give 2.25 g yellow solid 3, 5-dinitro-1, 2, 4-triazole potassium salt (DNPK) with a yield of 90.7%. 13C NMR (D2O-d2, 125 MHz) δ: 161.74; IR (KBr, ν/cm-1): 1620 (—C=N), 1568, 1383, 1316 (—NO2), 1497, 1036, 843 (triazole ring); Anal. Calcd for C2N5O4K: C 12.18, N 35.52; found C 12.30, N 35.43.
2.3.2 Synthesis of BDNTMN3, 5-Dinitro-1, 2, 4-triazole potassium salt(DNTK) (0.4 g, 2.03 mmol) was transferred into a three-necked round bottom flask, then 11.0 mL of N, N′-dimethyl formamide and 1, 3-dichloro-2-nitrazapropane (DCNP) (0.61 g, 3.86 mmol) were added at room temperature. The reaction mixture was stirred at 70-75 ℃ for 2 h, and then cooled to room temperature. The solution poured into ice water, the precipitate was filtered off, washed with cold water, and dried to give 0.37 g yellow solid BDNTMN with a yield of 90.2%. 1H NMR (DMSO-d6, 500 MHz) δ: 6.484 (s, 4H, 2CH2); 13C NMR (DMSO-d6, 125 MHz) δ: 62.395, 144.61; IR (KBr, ν/cm-1): 3044, 2986, 2927(—CH2), 1667(—C=N), 1564, 1385(—NO2), 1498, 1019, 842 (Triazole ring); MS (m/z): 404[M+]; Anal. calcd for C6H4N12O10: 17.83, N 41.59, H 1.00; found C 17.73, N 41.71, H 1.05.
2.4 Structure Determination of DNTSingle crystals of DNT suitable for X-ray diffraction studies were grown by allowing water to slowly diffuse into a saturated solution of DNT for ten days at room temperature.
A yellow single crystal with dimensions of 0.20 mm×0.18 mm×0.23 mm was chosen for X-ray diffraction analysis and the data were collected on a Bruker SMART APEXII CCD X-ray diffractometer with a MoKα radiation (λ=0.71073 Å) by using a
The single crystal of DNT is obtained for the first time in this paper (CCDC No. 920605). The selected bond lengths and bond angles, selected torsion angles and hydrogen bonds are given in Tables 1, 2 and 3, respectively. A displacement ellipsoid plot with atomic numbering scheme and a perspective view of the crystal in a unit cell are shown in Fig. 1 and Fig. 2, respectively.
| Tab.1 Selected bond lengths and bond angles of the DNT |
| Tab.2 Selected torsion angles |
| Tab.3 Hydrogen bond lengths and bond angles for DNT |
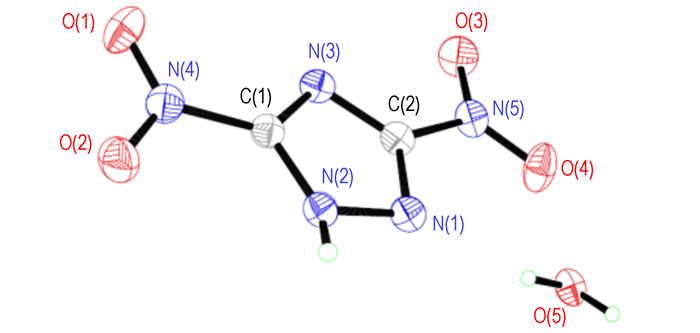
|
Fig.1 Molecular structure of DNT |
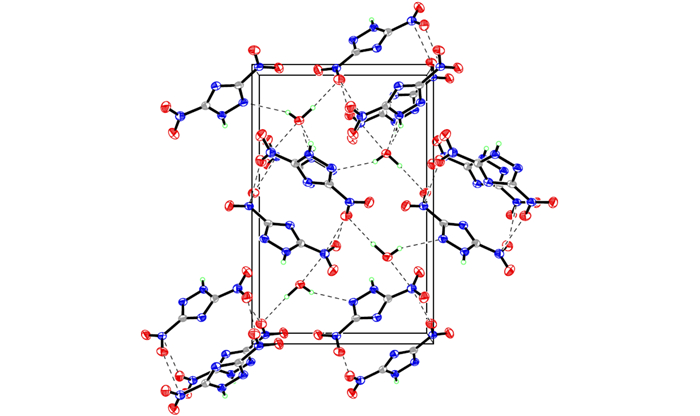
|
Fig.2 The packing of the DNT in unit cell |
It can be seen from Fig. 1 that the molecular structure of DNT is composed of one 1, 2, 4-triazole ring and two nitro groups to C(1) and C(2), respectively. Because of the Van Der Waals repulsion between those substituents contacted to C(1) and C(2), the torsion angles, which are O(2)—N(4)—C(1)—N(3) (177.7(2)), N(1)—N(2)—C(1)—N(3) (-0.2(3)), N(2)—N(1)—C(2)—N(5) (178.71(19)), O(4)—N(5)—C(2)—N(1) (0.6(3)), N(1)—N(2)—C(1)—N(4) (-179.3(2)), O(4)—N(5)—C(2)—N(3) (179.2(2)), O(2)—N(4)—C(1)—N(2) (-3.3(3)), O(3)—N(5)—C(2)—N(3) (-1.7(4)), in Table 4 indicate that all non-hydrogen atoms are almost in one plane. In addition, there are intermolecular O(5)—H(1)…O(3) (x+1, y, z), O(5)—H(1)…N(5) (x+1, y, z), N(2)—H(3)…O(5) (-x+3/2, -y+1, z+1/2) hydrogen bonds between DNT and H2O molecule, which further stabilize the structure. The corresponding lengths and angles of hydrogen bonds are listed in Table 3.
3.2 Thermal Behavior of BDNTMNThe DSC and TG-DTG analyses reveal that BDNTMN is thermally stable up to 150 ℃. The DSC curve of BDNTMN in Fig. 3 exhibits that the compound has no melting point, but exists two thermal decomposition peaks at 170.4 ℃ and 254.1 ℃, respectively. The TG-DTG curve of BDNTMN in Fig. 4 shows two-stage decomposition process with a mass loss of 15.66% before 180.3 ℃ at first stage decomposition and a total mass loss of 71.22% before 290.4 ℃ at the second stage decomposition, and a 16.36% residue at 496.1 ℃.
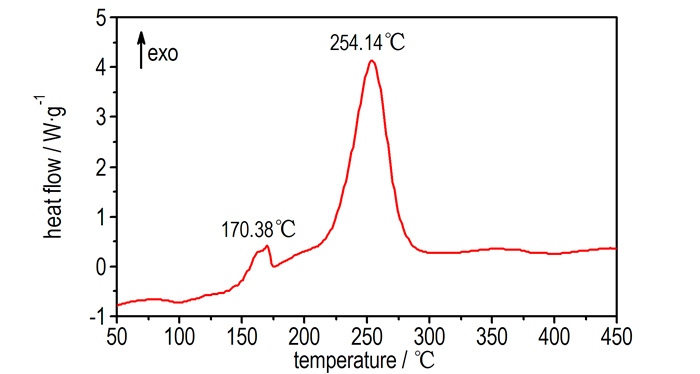
|
Fig.3 DSC curve of BDNTMN |
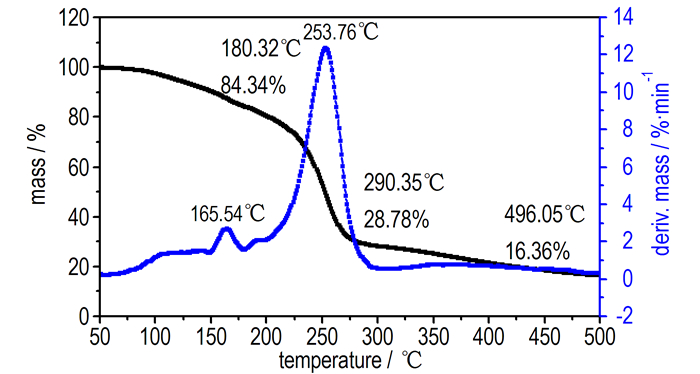
|
Fig.4 TG-DTG curve of BDNTMN |
All computations are performed using the Gaussian 09 suite of programs [14]. The elementary geometric optimization and the frequency analysis are carried out at the level of Becke three Lee-Yan-Parr (B3LYP) functionals with 6-31+G (d, p) basis set[15-17]. All of the optimized structures are characterized to be local energy minima on the potential surface without any imaginary frequencies.
The heat of formation (HOF) of the related compounds mentioned in this paper is determined by using the isodesmic reaction method. The isodesmic reaction of BDNTMN is carried out as described by Hakima Abou-Rachid (Scheme 2). Based on the optimized structure in Fig. 5, the total energy (E0) and thermodynamic parameters, including zero point energy (ZPE) and thermal correction to enthalpy (Hcorr), are obtained at MP2(full)/6-311++G(d, p) level, then the heat of the isodesmic reaction can be obtained.
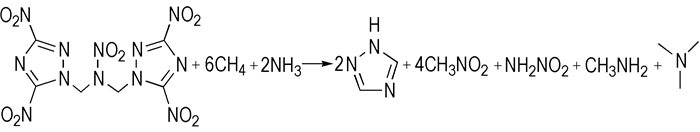
|
Scheme2 Isodesmic reaction for BDNTMN |
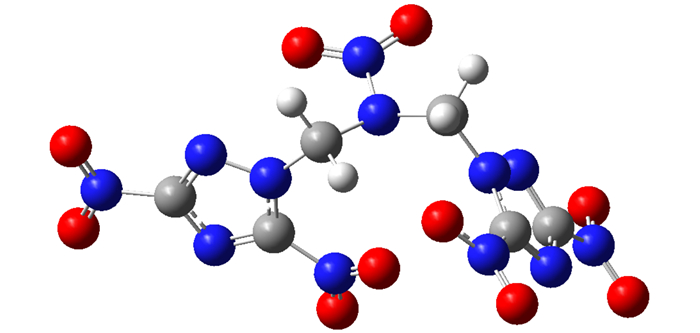
|
Fig.5 The optimized structure of BDNTMN at B3LYP/6-31+G(d, p) level |
The detonation parameters of BDNTMN are obtained by Kamlat-Jacbos equations[16] using the density and heat of formation as basic data. The performance data of BDNTMN, 1, 3, 5-trinitro-1, 3, 5-triazinane (RDX) and 1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetraazinane (HMX) are shown in Table 4. Results show that detonation performances of BDNTMN are better than those of RDX, similar with HMX.
| Tab.4 Properties of BDNTMN, RDX and HMX |
(1) N, N-bis((3, 5-dinitro-1H-1, 2, 4-triazol-1-yl)methyl)nitramide (BDNTMN) was synthesized using 3, 5-diamino-1, 2, 4-triazole as starting material via diazotization, nitration and N-alkylation reaction.
(2) BDNTMN has higher positive heat of formation, which is 425 kJ·mol-1 compared with 92.6 kJ·mol-1 for RDX and 104.8 kJ·mol-1 for HMX.
(3) BDNTMN has two stage decomposition process under heating condition, and two thermal decomposition peaks are at 170.4 ℃ and 254.1 ℃, respectively.
(4) The detonation performances of BDNTMN are better than those of RDX, similar with HMX.
| [1] |
Zhang Y, Huang Y, Parrish D A, et al. 4-Amino-3, 5-dinitropyrazolate salts—highly insensitive materials[J]. J Mater Chem, 2011, 21(19): 6891-6897. DOI:10.1039/c1jm10340g |
| [2] |
Dippold A A, Klapötke T M, Martin F A, et al. Nitraminoazoles based on ANTA—a comprehensive study of structural and energetic properties[J]. Eur J Inorg Chem, 2012, 14: 2429-2443. |
| [3] |
Gao H, Shreeve J M. Trinitroethyl—a functionality leading to energetic compounds with high nitro content[J]. RSC Adv, 2014, 4(47): 24874-24880. DOI:10.1039/c4ra03885a |
| [4] |
Klapötke T M, Petermayer C, Piercey D G, et al. 1, 3-Bis(nitroimido)-1, 2, 3triazolate anion, the N-nitroimide moiety, and the strategy of alternating positive and negative charges in the design of energetic materials[J]. J Am Chem Soc, 2012, 134(51): 20827-20836. DOI:10.1021/ja310384y |
| [5] |
LI Ya-nan, ZHANG Zhi-zhong, GE Zhong-xue, et al. Study of furoxan derivatives for energetic applications[J]. Chin J Chem, 2013, 31(4): 520-524. DOI:10.1002/cjoc.201201169 |
| [6] |
JIN Xing-hui, HU Bing-cheng, LIU Zu-liang, et al. Synthesis and properties of 1-amino-2-nitroguanidinium 4-nitroamino-1, 2, 4-triazole salt[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao), 2015, 23(3): 213-217. |
| [7] |
XU Cheng, BI Fu-qiang, ZHANG Min, et al. Synthesis, crystal structure and properties of diguanidinium 3, 3'-bis(nitramino)-4, 4'-azofurazanate[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao), 2015, 23(2): 199-201. |
| [8] |
Singh R P, Verma R D, Meshri D T, et al. Energetic nitrogen-rich salts and ionic liquids[J]. Angew Chem Int Ed, 2006, 45(22): 3584-3360. DOI:10.1002/(ISSN)1521-3773 |
| [9] |
Gao H, Shreeve J M. Azole-based energetic salts[J]. Chem Rev, 2011, 111(11): 7377-7436. DOI:10.1021/cr200039c |
| [10] |
YANG Wei, WANG Bo-zhou, WANG You-bin, et al. Synthesis of two novel explosives based on 1, 2-dinitroguanidine[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao), 2012, 20(5): 653-655. |
| [11] |
ZHANG Min, BI Fu-qiang, XU Cheng, et al. Synthesis and quantum chemistry calculation of bis[2-(5-nitrotetrazol-2-yl)-2, 2-dinitroethy ]nitramine[J]. Chinese Journal of Explosives & Propellants, 2014, 37(5): 52-57. |
| [12] |
Sheldrick G M. SHELXTS-97, Program for Crystal Structure Solution[CP]. University of Göttingen, Germany, 1997.
|
| [13] |
Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement[CP]. University of Göttingen, Germany, 1997.
|
| [14] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09. Revision B. 01[CP]. Gaussian, Inc, Wallingford CT, 2009.
|
| [15] |
Stephens P J, Devlin F J, Chabalowski C F, et al. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields[J]. J Phys Chem, 1994, 98(45): 11623-11627. DOI:10.1021/j100096a001 |
| [16] |
Hariharan P C, Pople J A. Influence of polarization functions on molecular-orbital hydrogenation energies[J]. Theor Chim Acta, 1973, 28(3): 213-222. DOI:10.1007/BF00533485 |
| [17] |
Hehre W J, Radom L, Schleyer P V R, et al. Ab Initio Molecular Orbital Theory[M]. John Wiley and Sons, New York, USA, 1985.
|
| [18] |
Mayer R, Köhler J, Homburg A. Explosive[M]. 6th ed. Weinheim, Germany, Wiley-VCH. 2007.
|
| [19] |
Wang R H, Xu H Y, Guo Y, et al. Bis[3-(5-nitroimino-1, 2, 4-triazolate)]-based energetic salts: synthesis and promising properties of a new family of high-density insensitive materials[J]. J Am Chem Soc, 2010, 132(34): 11904-11905. DOI:10.1021/ja1055033 |
N, N-bis((3, 5-dinitro-1H-1, 2, 4-triazol-1-yl)methyl)nitramine (BDNTMN) was synthesized via diazotization, nitration and N-alkylation etc reactions using 3, 5-diamino-1, 2, 4-triazole (DAT) as starting material. The structures of each compound were characterized by means of 1H NMR, 13C NMR, IR, MS and elemental analysis. The thermal behaviors of BDNTMN were analyzed by DSC and TG. The physicochemical property and detonation performance of BDNTMN were predicted by Gaussian 09 and Kamlat-Jacbos equations.




