2. State Key Laboratory of Fluorine & Nitrogen Chemical, Xi′an 710065, China
2. 氟氮化工资源高效开发与利用国家重点实验室, 陕西 西安 710065
The demand for high-performance explosives and propellants has led to intensive investigations relative to improving their energetic, mechanical, and storage properties and safe handling. One of the key steps is the development of new energetic ingredients, such as energetic plasticizers. Energetic plasticizers are important constituents of rocket and gun propellants, which aid in improving the mechanical properties of the propellant grain[1-5].The basic objectives of developing energetic plasticizers are: (1) increase of the thermal stability, (2) increase in energy content, (3) adjustment of the oxygen balance in a formulation, (4) improvement of the plasticizer functions in formulations. Furazanyl ether compounds have attracted a considerable amount of attention as an energetic plasticizers due to their high energy density, good thermal stability and positive heat of formation [6-14]. When a bridged oxygen atom is introduced into furazans, it could be significantly increased both the flexibility and plasticizer of the molecule. A good example is the previously synthesized compound 3, 3′-dinitrodifurazanyl ether (FOF-1), which shows a low melting point (Tm=63-64 ℃), high density (ρ=1.90 g·cm-3) and good thermal stability (Tdec>200 ℃).
In order to raise the thermal stability and energy level of the energetic plasticizer, introducing furazanyl ether as backbone into fluorodinitromethyl group could be an effective method. Chemically, the substitution of one nitro group in the trinitromethyl moiety with a fluorine atom reduces the pseudohalide character[15]. In view of the observations above, a detailed study of the synthesis and characterization of 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan is presented in this work. In addition, thermal stability, sensitivity measurements and the detonation parameters were investigated.
2 Experimental 2.1 Instruments and ConditionsElemental analyses of C, H and N were performed on a VARI-El-3 elementary analysis instrument. Infrared spectra were obtained from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Differential scanning calorimetry(DSC) studies were carried out on a Q200 apparatus(TA, USA) with a heating rates of 10 K·min-1, using dry oxygen-free nitrogen as atmosphere with a flowing rate of 50 mL·min-1. The TG-DTG experiment was performed with a SDT-Q600 apparatus(TA, USA) operating at a heating rate of 10 K·min-1 in a flow of dry oxygen-free nitrogen at 50 mL·min-1. The density was measured using a pycnometer method at room temperature. The impact sensitivity was determined with a ZBL-B impact sensitivity instrument. The heat of formation for 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy) furazan was theoretically computed using the Gaussian 09 (Revision A.02) program package[16]. To obtain very accurate energies, the enthalpies (H) were calculated by using the CBS-4M method[17-18].
Dipotassium of 3, 4-bis(3-dinitromethylfurazan-4-oxy) furazan (2) was prepared according to the published procedures[19]. Other chemicals were obtained from commercial sources and used without further purification.
2.2 Synthesis
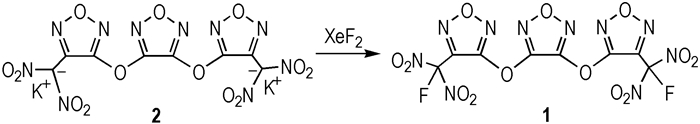
|
Scheme1 |
3, 4-Bis(3-fluorodinitromethylfurazan-4-oxy) furazan(1): To a suspension of dipotassium 3, 4-bis(3-dinitromethylfurazan-4-oxy)furazan (0.35g, 0.67mmol) in an hydrous acetonitrile (15 mL) was added XeF2 (0.46 g, 2.7 mmol) with stirring and reaction mixture was stirred for 20 h at 30 ℃. The acetonitrile was evaporated, and the residue was treated with water. The white crystals was collected by filtration to afford 1 (0.18 g, 56.2%) as a colorless crystals. mp 50 ℃; 13C NMR (DMSO-d6, 125 MHz) δ 159.85, 153.33, 138.03, 114.21; 19F NMR (DMSO-d6, 470.5MHz) δ: -106.84; IR (KBr, ν/cm-1) 1608, 1576, 1545, 1507, 1350, 1309, 1250, 1194, 1033, 982; Anal.Calcd. for C8N10O13F2 (%): C 19.93, N 29.05; Found C 20.08, N 29.13.
3 Physicochemical and Energetic PropertiesThe physicochemical and energetic properties of 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy) furazan were determined or calculated, and listed in Table 1. It was found that 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan possesses a good thermal stability with decomposition temperature of 197.8 ℃, moderate impact sensitivity of 11 J. The density of 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan is 1.88 g·cm-3. The oxygen balance is calculated to be -6.6%, and this value is lower than that of NG (3.5%), but higher than that of FEFO(-10%).
| Tab.1 The physicochemical properties and detonation parameters of 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan compared with the well-known plasticizers NG, FEFO and FOF-1 |
Based on the calculated heats of formation and the experimentally measured densities, the detonation pressures and detonation velocities were calculated based on Kamlet-Jacobs equations and shown in Table 1. Compound 1 has a detonation velocity of 8644.5 m·s-1 and a detonation pressure of 34.0 GPa, which are significantly better than those of nitroglycerine(NG) (25.7 GPa, 7813 m·s-1)[20] and bis(2-fluoro-2, 2-diniroethyl)formal (FEFO)(25 GPa, 7500 m·s-1)[21], and comparable with detonation velocity of 3, 3′-dinitrodifurazanyl ether (FOF-1) (8930 m·s-1)[22], revealing a higher energy level as a new high-performance energetic plasticizer.
4 ConclusionsIn this study we reported the synthesis and structural property, spectroscopic characterization and detonation performances of 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan (1). Compound 1 exhibits good physicochemical and detonation properties, such as high density(1.88 g·cm-3), good thermal stability (Tdec=197.8 ℃), moderate impact sensitivity(11 J), acceptable oxygen balance(-6.6%), and high detonation pressure(34.0 GPa) and detonation velocity(8644.5 m·s-1). In many aspects, such as thermal stability, density, sensitivity, and detonation parameters, it is far superior to NG. This promising result makes the 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan interesting for future application as a potential high-energy dense plasticizer.
| [1] |
Kumari D, Balakshe R, Banerjee S, et al. Energetic plasticizers for gun & rocket propellants[J]. Review Journal of Chemistry, 2012, 2(3): 240-262. DOI:10.1134/S207997801203003X |
| [2] |
Klapötke T M. Chemistry of High Energy Materials[M]. Berlin/New York: Walter de Gruyter, 2012.
|
| [3] |
Chen Y, Kwon Y, Kim J S. Synthesis and characterization of bis(2, 2-dinitropropyl ethylene) formal[J]. Journal of Industrial and Engineering Chemistry, 2012, 18: 1069-1075. DOI:10.1016/j.jiec.2011.12.006 |
| [4] |
CHEN Bin, ZHANG Zhi-zhong, JI Yue-ping. Synthesis and application of gem dinitro energetic plasticizers[J]. Chinese Journal of Explosives & Propellants, 2007, 30: 67-70. |
| [5] |
Provatas A. Energetic plasticizer migration studies[J]. Journal of Energetic Materials, 2003, 21: 237-245. DOI:10.1080/713770435 |
| [6] |
WANG Bo-zhou, ZHAI Lian-jie, LIAN Peng, et al. A novel synthesis of 3, 3-bis(fluorodinitromethyl) difurazanyl ether(FOF-13)[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2014, 22(6): 884-886. |
| [7] |
WANG Wei, ZHAI Lian-jie, HUO Huan, et al. Synthesis and crystal structure of 3, 4-bis(3-chlorodinitromethylfurazan-4-oxy)furazan[J]. Chemical Reagents, 2015, 37: 200-204. |
| [8] |
Sheremetev A B. Chemistry of furazans fused to five-membered rings[J]. Journal of Heterocyclic Chemistry, 1995, 32: 371-385. DOI:10.1002/jhet.v32:2 |
| [9] |
Badgujar D M, Talawar M B, Asthana S N, et al. Advances in science and technology of modern energetic materials: an overview[J]. Journal of Hazardous Materials, 2008, 151: 289-305. DOI:10.1016/j.jhazmat.2007.10.039 |
| [10] |
Sheremetv A B, Mantseva E V. Hydroxyfurazans: outlook to using[C]//32th International Annual Conference of ICT, Karlsruhe, Germany. 2001, 103: 1-4.
|
| [11] |
Sheremetev A B, Kulagina V O, Aleksandrova N S, et al. Dinitro trifurazans with oxy, azo and azoxy bridges[J]. Propellants, Explosives, Pyrotechnics, 1998, 23: 142-149. DOI:10.1002/(ISSN)1521-4087 |
| [12] |
Sheremetev A B. 3, 3-Bis(1-fluoro-1, 1-dinitromethyl)difurazanyl ether[C]//29th International Annual Conference of ICT, Karlsruhe, Germany, 1998, 58: 1-6.
|
| [13] |
FAN Yan-jie, WANG Bo-zhou, LAI Wei-peng, et al. Synthesis, characterization and quantum chemistry study on 3, 3′-dicyanodifurazanyl ether (FOF-2)[J]. Chinese Journal Organic Chemistry, 2009, 29(4): 614-620. |
| [14] |
ZHOU Yan-shui, XU Kang-zheng, WA NG, Bo-zhou, et al. Synthesis, structure and thermal properties of bifurazano[3, 4-b :3′, 4′-f] furoxano [3″, 4″-d] oxacyclohetpatriene (BFFO)[J]. Bulletin of the Korean Chemical Society, 2012, 33: 3317-3320. DOI:10.5012/bkcs.2012.33.10.3317 |
| [15] |
Patai S. The chemistry of functional group: the chemistry of the carbon-halogen Bond: Part 1[M]. London: J Wiley & Sons Ltd., 1973.
|
| [16] |
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, rev. A. 02[CP], Gaussian, Inc., Wallingford CT, 2009.
|
| [17] |
Montgomery Jr J A, Frisch M J, Ochterski J W, et al. A complete basis set model chemistry. Ⅶ. Use of the minimum population localization method[J]. The Journal of Chemistry Physics, 2000, 112(5): 6532-6542. |
| [18] |
Ochterski J W, Petersson G A, Montgomery Jr J A. A complete basis set model chemistry. Ⅴ. Extensions to six or more heavy atoms[J]. The Journal of Chemistry Physics, 1996, 104(7): 2598-2619. DOI:10.1063/1.470985 |
| [19] |
ZHAI Lian-jie, FAN Xue-zhong, WANG Bo-zhou, et al. A green high-initiation-power primary explosive: synthesis, 3D structure and energetic properties of dipotassium 3, 4-bis(3-dinitromethylfurazan-4-oxy)furazan[J]. RSC Advances, 2015, 5: 57833-57841. DOI:10.1039/C5RA09822J |
| [20] |
Meyer R, Kohler J, Homburg A. Explosives[M]. Fifth Edition. Weinheim: VCH, 2002.
|
| [21] |
Karl K, Renato R. Development of an efficient to manufacture bis (2-fluoro-2, 2-diniroethyl) formal (FEFO)[C]//18th International Annual Conference of ICT, Karlsruhe, Germany, 1987, 28: 1-5.
|
| [22] |
WANG Bo-zhou, LI Hui, LI Ya-nan, et al. Review on energetic compounds based on furoxanyl ether[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(4): 385-390. |
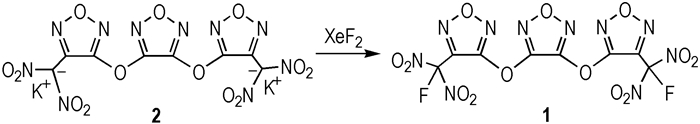
An excellent energetic plasticizer 3, 4-bis(3-fluorodinitromethylfurazan-4-oxy)furazan was self-designed and synthesized, and its main energetic properties were investigated.




