Triazole energetic compound is a kind of interesting nitrogen-rich energetic materials owing to their unique characteristics, such as high nitrogen content, high positive enthalpy and strong power, and are extensively applied to the field of energetic materials, such as insensitive high explosives, propellants and pyrotechnics[1-2].Recently, there are many information about triazole energetic compounds in the available literature, for example, 1, 2, 4-triazole[3-11], 1, 2, 3-triazole[12-16] and their derivatives.However, triazole ionic salts have been paid much more attention owing to higher density and energy, better thermal stability than those of non-ionic salts.
Hydrazinium 3, 5-dinitroamino-1, 2, 4-triazole(HDNAT) is a new and unexplored triazole ionic salt compound(scheme 1) and exhibits excellent thermal stability(194-196 ℃), high nitrogen content(57.01%), high density(1.89 g·cm-3) and detonation velocity(9000 m·s-1 by 1.80 g·cm-3).HDNAT can be applied to the field of propellants and gas-forming agents, especially propellants in order to increase burning rate.
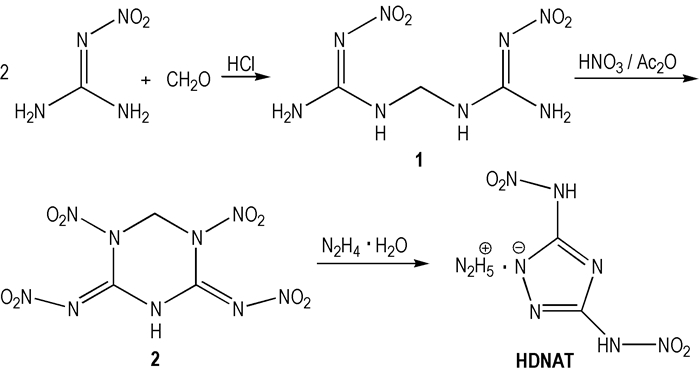
|
Scheme1 The synthetic route of HDNAT |
Using nitroguanidine and formaldehyde as starting materials, HDNAT was firstly designed and synthesized by condensation, nitrification and hydrazinolysis reaction(Scheme 1), HDNAT and its intermediates were characterized by the means of NMR, IR and elemental analysis.The properties of physico-chemistry and detonation for HDNAT were obtained by tests and calculation.
2 Experimental 2.1 Synthesis of bis(nitroguanidine) methane(1)Nitroguanidine(10.4 g, 0.10mol), formaldehyde(4.0 mL, 0.05 mol) was added into 60.0 mL of 10% hydrochloric acid at room temperature and the mixture was heated for 1h at 70 ℃.The solution was cooled to 20 ℃, and the white precipitate was filtered to obtain 10.6 g solid with a yield of 96.4%.1H NMR(DMSO-d6, 500 MHz), δ:8.1487(s, 4H, 2NH2), 4.5965(s, 2H, 2NH), 3.3488(s, 2H, CH2); 13C NMR(DMSO-d6, 125 MHz), δ:159.081, 72.858;IR(KBr, ν/cm-1):3420, 3330, 3253, 3118, 2988, 1661, 1596, 1520, 1295, 896;Anal.calcd for C3H8N8O4:C 16.36, H 3.64, N 50.91;found C 16.46, H 3.75, N 51.10.
2.2 Synthesis of 2, 4-dinitroimino-1, 5-dinitro-1, 3, 5-triazine(2)Bis(nitroguanidine) methane(1)(5.0 g, 0.023 mol) was added in batches in nitric acid(24 mL, 100%) and Ac2O(24 mL, 100%) at 0-5 ℃, then the mixture was heated for 3 h at 20 ℃.After 30 min the white precipitate was formed, which was removed by filtration, washed by dichloromethane to obtain 4.8 g solid with a yield of 71.2%, m.p.:100-104 ℃(dec.).1H NMR(DMSO-d6, 500 MHz), δ:12.7553(s, H, NH), 6.2888(s, 2H, CH2); 13C NMR(DMSO-d6, 125 MHz), δ:148.557, 58.998;IR(KBr, ν/cm-1):3169, 3061, 2974, 1654, 1557, 1396, 1296, 899;Anal.calcd for C3H3N9O8:C 12.29, H 1.02, N 43.00;found C 12.52, H 1.32, N 43.21.
2.3 Synthesis of HDNAT2, 4-Dinitroimino-1, 5-dinitro-1, 3, 5-triazine(2)(4.0 g, 13.6 mmol) was dissolved in 40 mL water and hydrazine hydrate(1.8 mL, 27.2 mmol) was added dropwise.The mixture was heated for 30 min at 30 ℃ and the solution was cooled to 10 ℃, and the white precipitate was filtered to obtain 2.8 g solid with a yield of 92.8%, m.p.:194-196 ℃(dec.).1H NMR(DMSO-d6, 500 MHz), δ:7.9228, 13.4263;13C NMR(DMSO-d6, 125 MHz), δ:150.937;IR(KBr, ν/cm-1):3319, 3180, 1586, 1542, 1367, 1253, 983, 719;Anal.calcd for C2H7N9O4:C 10.86, H 3.17, N 57.01;found C 11.09, H 3.26, N 57.37.
2.4 Properties of HDNATTable 1 is some properties of HDNAT.The properties of physico-chemistry and detonation for HDNAT, including the appearance, density, dissolubility and melting point were obtained by the tests.Its detonation properties, such as friction sensitivity, impact sensitivity, H50 and detonation velocity were obtained by national standard method, and detonation pressure was calculated by VLW method[17].
| Tab.1 Properties of HDNAT |
Hydrazinium 3, 5-dinitroamino-1, 2, 4-triazole(HDNAT), a novel triazole ionic salt compound, was synthesized for the first time with a total yield of 63.69% and high nitrogen content of 57.01%, a high density 1.89 g·cm-3 and a high detonation velocity 9000 m·s-1 and a excellent thermal stability.However, HDNAT shows high sensitivity(friction sensitivity of 92%, impact sensitivity of 100% and H50 of 26.8 cm), and might be potentially useful as propellants.
| [1] |
LI Guan-qiong, LI Yu-chuan, MA Qiao-li, et al. Research progress in synthesis of nitrogen-rich zole-ring compounds by cycloaddition reaction[J]. Chinese Journal of Organic Chemistry, 2010, 30(10): 1431-1440. |
| [2] |
LIU Xiao-jian, ZHANG Hui-juan, LIN Qiu-han, et al. Progress of study on the synthesis of azole energetic ionic compounds[J]. Chinese Journal of Explosives & Propellant, 2010, 33(1): 6-10. |
| [3] |
Lee K, Storm C, Hiskey M, et al. An improved synthesis of 5-amino-3-nitro-1H-1, 2, 4-triazole(ANTA), a useful intermediate for the preparation of insensitive high explosives[J]. Journal of Energetic Materials, 1991, 9(5): 415-428. DOI:10.1080/07370659108019382 |
| [4] |
WANG Xi-jie, JIA Si-yuan, WANG Bo-zhou, et al. Synthesis improvement of 5-amino-3-nitro-1, 2, 4-triazole(ANTA)[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2006, 14(6): 439-440. |
| [5] |
ZHU Zhao-yang, CAO Yi-lin, SUN Zhong-xiang, et al. Investigation on synthesis and properties of 5, 5'-dinitro-3, 3'-azo-1-hydro-1, 2, 4-triazole(DNAT)[J]. Journal of Solid Rocket Technology, 2008, 31(5): 501-503. |
| [6] |
XIA Yun-xia, WANG Ping, SUN Jie, et al. Synthesis and properties of 4-amino-1, 2, 4-triazole picrate[J]. Fine Chemicals, 2008, 25(5): 445-448. |
| [7] |
ZHANG Hai-hao, JIA Si-yuan, WANG Bo-zhou, et al. Synthesis and characterization of 3-amino(nitro)-5-nitro-1, 2, 4-triazole derivatives[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2010, 18(1): 29-33. |
| [8] |
HE Yun, FAN Gui-juan, ZHANG Guang-quan, et al. Review on synthesis and reactivity of 5-amino-3-nitro-1, 2, 4-trizole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(6): 715-720. |
| [9] |
ZHOU Qun, WANG Bo-zhou, JIA Si-yuan. New synthetic of 1-methyl-3-amino-5-nitro-1, 2, 4-triazole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(1): 26-29. |
| [10] |
LI Lei, CHI Yu, ZHANG Yong, et al. Synthesis, characterization and crystal structure of 4, 4'-bis-1, 2, 4-trizole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2013, 21(4): 429-433. |
| [11] |
XU Hai-feng, WANG Juan, LI Yong-qiang, et al. Synthesis of 2, 4, 6-trinitro-3, 5-diamino-N-(1, 2, 4-trizole-4)-aniline[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2013, 21(6): 721-725. |
| [12] |
LUO Yi-fen, CHEN Xiao-fang, WANG Bo-zhou, et al. Synthesis, thermal performance and quantum chemistry study on 4-nitro-2-(1-nitro-1, 2, 4-triazole-3-yl)-1, 2, 3-triazole(DNDTz)[J]. Chinese Journal of Explosives & Propellant, 2013, 36(4): 13-17. |
| [13] |
HUO Huan, WANG Bo-zhou, ZHOU Cheng, et al. Synthesis and characterization of 4-amino-5-nitro-1, 2, 3 -triazole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2008, 16(1): 49-52. |
| [14] |
WANG Bo-zhou, LI Ji-zhen, HUO Huan, et al. Synthesis, characterization and thermal behaviors of 4-amino-5-nitro-1, 2, 3-triazole(ANTZ) and its derivatives[J]. Chin J Chem, 2010, 28: 781-784. DOI:10.1002/cjoc.v28:5 |
| [15] |
SHI Hong-gang, LI Sheng-hua, LI Yu-chuan, et al. Synthesis of l-amino-1, 2, 3-triazole[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2008, 16(6): 676-678. |
| [16] |
LI LIN, YE Zhi-wen, Lü Chun-xu. Synthesis and characterization of 1-amino-3-methyl-1, 2, 3-triazolium nitrate[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2012, 20(1): 9-12. |
| [17] |
WU Xiong, LONG Xin-Ping, HE Bi, et al. The VLW equation of state for detonation products[J]. Science in China, 2008, 38(12): 1129-1131. |
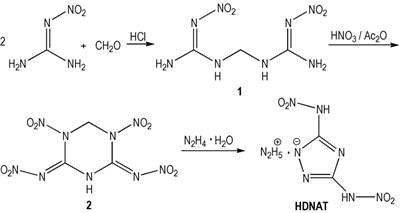
A novel energetic material,hydrazinium 3,5-dinitroamino-1,2,4-triazole(HDNAT) was designed and synthesized for the first time via condensation,nitrification and hydrazinolysis reaction.In addition,some main properties of physico-chemistry and detonation for HDNAT were obtained by test or calculation.



