- Online First |
- Articles in press |
- Current Issue |
- Special Articles |
- Archive
-
Online:June 13, 2025 DOI: 10.11943/CJEM2025044
Abstract:Carotenoids, valued for their exceptional free radical scavenging properties and low biological toxicity, were systematically investigated as potential stabilizers for propellants. A comprehensive evaluation strategy, incorporating differential thermal analysis (DTA), methyl violet test strips, isothermal thermogravimetry, vacuum stability testing, and accelerating rate calorimetry (ARC), was employed to assess their stabilizing effects. Four representative carotenoids-lycopene, β-carotene, xanthophyll, and astaxanthin, were examined for their stabilization performance in nitrocellulose (NC) and absorptive composition systems. All tested carotenoids demonstrated superior thermal stability compared to conventional stabilizers. Notably, astaxanthin exhibited the most significant enhancement: it prolonged the methyl violet discoloration time of NC by 40 min, reducing mass loss by 17.90%, decreased the maximum adiabatic decomposition temperature rise rate by 0.134 ℃·min-1, and lowered gas pressure release per unit mass by 12.0 kPa. In absorptive compositions, it extended the methyl violet discoloration time by 34 min while reducing mass loss by 14.18%. Free radical scavenging tests and intermediate structural analyses revealed the underlying stabilization mechanism: carotenoids effectively suppress autocatalytic decomposition via nitrogen-oxygen free radical capture, achieving nearly 90% scavenging efficiency at 8 mmol·L-1. Additionally, secondary derivatives formed during carotenoid degradation were free of nitrosamine groups, significantly reducing toxicological concerns.
-
LIU Ding, ZHANG Yan, NIU Shi-yao, ZHAO Feng-qi, LI Si-heng, DONG Ying-nan, QU Wen-gang
Online:July 10, 2025 DOI: 10.11943/CJEM2025016
Abstract:The combustion process of energetic materials (EMs) is a complex multi-stage process. By studying their thermal decomposition and combustion reactions, establishing precise combustion reaction kinetics models enables effective prediction of the thermal behavior of EMs, which is of significant importance for their synthesis, production, transportation, storage, and practical application in modern weaponry and equipment. Compared to traditional EMs, third-generation EMs exhibit higher energy density, which imposes more stringent requirements on their thermal stability. This review summarizes recent advances in thermal properties and combustion research of third-generation EMs, including both ionic and covalent types. The current research status on thermal properties and combustion reactions of typical third-generation EMs is expounded from three perspectives: thermal decomposition profiles, decomposition pathways/mechanism, and combustion performance. It identifies the shortcomings of the current research and proposes the research direction of the thermal behavior of the third-generation energetic materials. It is proposed to construct a multi-scale coupled research system: high-precision measurement of combustion parameters via novel experimental apparatus, accurate diagnosis of combustion intermediates, and cross-scale modeling combining quantum chemistry-machine learning-fluid mechanics to achieve full-chain analysis from free-radical mechanisms to macroscopic flame propagation.
-
FAN Chao, LI Bo-hao, ZHANG Peng-chao, WEI Zong-liang, QIN Neng, MA Ning, XIE Zhong-yuan
Online:July 25, 2025 DOI: 10.11943/CJEM2025055
Abstract:To enhance the understanding of safety of multi-chamber mixing processes, a multiphase flow CFD numerical model based on the Eulerian method was established for the continuous mixing of multi-component materials in a multi-chamber kneader, taking a cast polymer bonded explosive (PBX) as the object. Experimental verification was conducted to confirm the reliability of the model. Based on the model, the influence laws of key process and structural parameters including blade rotation speed, kneading clearance and blade profile on the mixing safety stimulus were studied. The results show that the pressure level gradually decreased from the feeding chamber to the discharging chamber. Increasing the blade rotation speed was beneficial for reducing the pressure in the chambers, but the shear stimulus significantly increased. As the blade rotation speed increased from 15 r·min-1 to 75 r·min-1, the peak pressure in the kneader decreased from 402966 Pa to 258107 Pa, and the peak shear stress increased from 6268.5 Pa to 16607.9 Pa. Increasing the kneading clearance significantly reduced the pressure and shear stress in the chambers. As the kneading clearance increased from 1 mm to 5 mm, the peak pressure in the kneader decreased from 391094 Pa to 284478 Pa, and the peak shear stress decreases from 8320.5 Pa to 3982.6 Pa. Compared with the two-wing-two-wing blades, four-wing-two-wing blades produced stronger shear stimulus due to more kneading sites, but the blade profile had a smaller impact on the kneading pressure. When the four-wing-two-wing blades and two-wing-two-wing blades were used in chambers 1-7, the peak shear stresses in the kneader were 7481.3 Pa and 4518.1 Pa, respectively.
-
Abstract:
-
Abstract:
-
PENG Pan-pan, HU Xiang, TU Long-xiao, XIA Wen-tao, CAO Yan-jun, LIU Yu-jun, YANG Er-gang, ZHOU Shui-ping, TAO Bo-wen
2025,33(8):820-828, DOI: 10.11943/CJEM2025099
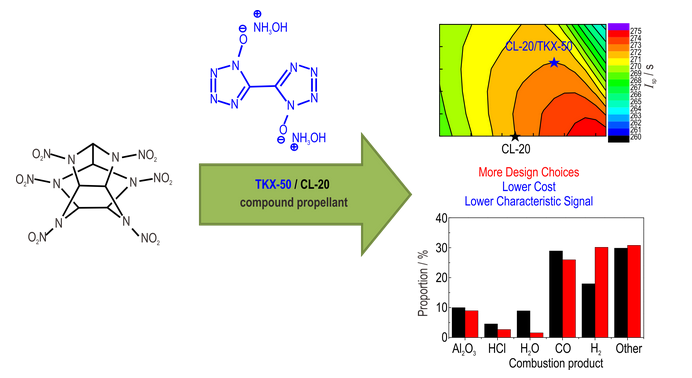
Abstract:To further explore the application potential of novel dinitramide energetic ionic salt (DBDN) with non-hygroscopic property, the synthetic route of DBDN was optimized. The standard molar enthalpy of formation (ΔfHθm) was calculated according to the measured constant volume heat of combustion (Qv). The energetic properties of DBDN in composite modified double base (CMDB) propellant and low signature propellant were calculated by RAMJ software. The kinetics of thermal decomposition of ammonium perchlorate (AP) catalyzed by DBDN were studied by Kissinger equation. Results indicate that the optimized synthetic conditions for DBDN are as follows: reaction temperature of 65 ℃, solvent of CH3CH2OH /H2O (volume ratio 10∶1), and reaction time of 5 hours. The Qv and ΔfHθm values of DBDN are -(13525.5±3.28) J·g-1 and -(17.36±0.24) kJ·mol-1, respectively. The calculation results show that the DBDN replaces 8% to 14% of AP, the performance of CMDB propellant is significantly improved. In the formulation of low signature propellant, DBDN has the ability to reduce the combustion chamber temperature (Tc) and the tail flame temperature (Te) by 17 times and 11 times larger than that of ADN respectively, and there is also no HCl gas in the combustion products. However meanwhile, DBDN weakens the energy performance of the propellants to some extent. In addition, the Kissinger equation shows that the addition of 10% mass fraction of DBDN decreases the activation energy of thermal decomposition of AP by 27.5 kJ mol-1, which is beneficial to catalyze the thermal decomposition of AP at high temperature, indicating that DBDN has the potential to adjust the burning rate and pressure exponent.
-
LAN Xiang-tao, ZHANG Ping-an, YUAN Jian-min, DENG Jian-ru
2025,33(8):829-835, DOI: 10.11943/CJEM2025132
Abstract:To achieve uniform structural composition of neutral macromolecular bonding agents and enhance their comprehensive performance, a novel monomer, cyanoethyl acrylate (CEA), was designed and synthesized. Through free radical copolymerization with hydroxyethyl acrylate (HEA), a new type of neutral macromolecular bonding agent was prepared. The structures of monomers and polymers were characterized by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR), and the hydroxyl value and glass transition temperature (Tg) were determined. The bonding agent was applied in NEPE high-energy propellant formulations to investigate its impact on the process and mechanical properties. Results show that CEA and HEA exhibit matched polymerization reactivity, enabling the production of structurally homogeneous copolymers without controlling the feed method. Compared to traditional neutral bonding agents, this bonding agent reduces the yield value of propellant slurry to 74.4 Pa and apparent viscosity to 337 Pa·s, making a notable improvement of processing performance. The propellant grain achieves tensile strength of 0.98 MPa and maximum elongation of 70.1% at ambient temperature, representing 22.5% and 27.5% improvements respectively.
-
XU Yi-qing, YANG Wei-juan, ZHANG Fan, LIU Jian-zhong, FAN Jian-ren
2025,33(8):836-846, DOI: 10.11943/CJEM2025091
Abstract:为了研究铝粉粒径和凝胶剂浓度对含能凝胶燃料流变性能的影响规律,以双丙酮-D-甘露糖醇为低分子凝胶剂,改变凝胶剂浓度、铝粉粒径及添加量,制备了系列JP-10基含能凝胶燃料。采用旋转流变仪系统测试了凝胶的稳态剪切特性、触变回复性能和动态振荡特性等,分析了凝胶剂浓度、铝粉浓度、铝粉粒径、温度以及表面活性剂浓度对体系流变行为的作用机制。结果表明,凝胶具有显著的剪切变稀特性,当剪切速率从0.01 s-1增至100 s-1时,表观黏度下降约4个数量级。提高凝胶剂浓度可同步增强凝胶的剪切黏度、触变回复率及屈服应力,使模量的频率依赖性增强。添加铝粉会明显增加凝胶黏度和松弛时间,且纳米铝粉的影响较微米铝粉更明显,并随着铝粉浓度的增加而提升。此外,表面活性剂和温度对凝胶流变性能也有一定的影响。随着表面活性剂浓度和温度的升高,凝胶体系的黏度、储能模量及耗能模量均呈现下降趋势。
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
-
QIN Yuan, PU Rui, TU Long-xiao, YAN Qi-long
2025,33(8):847-859, DOI: 10.11943/CJEM2025053
Abstract:To enhance the mechanical properties of HTPB four-composite solid propellants, 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane (A1130) and N-(Triethoxysilylpropyl)urea (A1160) were employed to modify the surface of HMX and qy-HMX, followed by their application in solid propellant formulation. Scanning electron microscope (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), atomic force microscope (AFM) and thermal analysis (DSC-TG) were used to test the morphology, structure and performance of samples. The interfacial enhancement effects were systematically investigated using an electronic universal testing machine and dynamic thermomechanical analyzer (DMA) to assess mechanical properties and adhesion characteristics. Results demonstrate that the silane treatment forms continuous coating layers without changing the crystalline structure. Silane coating inhibits effectively the transformation of HMX, increasing the phase transition temperature of HMX@A1130 and HMX@A1160 to 193.9 ℃ and 201.4 ℃, which are 2.3 ℃ and 9.8 ℃ higher than that of raw HMX. The mechanical tests reveal significant improvements in propellant tensile strength across both high temperature (70 ℃) and low temperature (-50 ℃) conditions. Notably, A1130-modified propellant exhibits an enhanced tensile strength with the adhesion index reduced from 1.52 to 1.24 at -50 ℃/500 mm·min-1. The tensile strength of propellants modified with HMX@A1130 and HMX@A1160 increases by 29.9% and 31.6%, and the maximum elongation increase by 29.9% and 31.6%, respectively. DMA results show that the peak value of loss factor for the A1130-modified propellant decreases from 0.51 to 0.47, indicating a mitigation of the interfacial ‘dewetting’ phenomenon at low temperatures. The fracture surface morphology analysis results are in good agreement with the tensile test and DMA test. The addition of two silane coupling agents has a significant interfacial modification effect, and A11330 can inhibit the interfacial ‘dewetting’ on hydroxyl-terminated polybutadiene system.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
- 136
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- 144
- 145
- 146
- 147
- 148
- 149
- 150
- 151
- 152
- 153
- 154
- 155
- 156
- 157
- 158
- 159
- 160
- 161
- 162
- 163
- 164
- 165
- 166
- 167
- 168
- 169
- 170
- 171
- 172
- 173
- 174
- 175
- 176
- 177
- 178
- 179
- 180
- 181
- 182
- 183
- 184
- 185
- 186
- 187
- 188
- 189
- 190
- 191
- 192
- 193
- 194
- 195
- 196
- 197
- 198
- 199
- 200
- 201
- 202
- 203
- 204
- 205
- 206
- 207
- 208
- 209
- 210
- 211
- 212
- 213
- 214
- 215
- 216
- 217
- 218
- 219
- 220
- 221
- 222
- 223
- 224
- 225
- 226
- 227
- 228
- 229
- 230
-
WANG Yu-feng, LI Gao-chun, LI Jin-fei, ZHU Zi-xiang, YUAN Mao-de, LI Xu, LI Yong-qiang
2025,33(8):860-866, DOI: 10.11943/CJEM2025074
Abstract:The adhesive interface of solid rocket motors is a weak zone prone to interfacial debonding and cohesive failure. Analyzing the meso-scale damage evolution process of adhesive interfaces is fundamental for motor failure assessment. Using an in-situ loading device and a high-resolution micro-CT system, in-situ scanning imaging tests were conducted on the adhesive interface of insulation layer/liner/propellant. The internal three-dimensional micro-digital images were obtained, and the meso-damage modes and evolution processes were analyzed. The digital volume correlation (DVC) method was employed to calculate the deformation fields of the adhesive interface under in-situ tensile conditions. The results show that under external loading, the deformation near the liner/propellant interface is greater than that in other regions. When the external strain reaches 6%, microvoids form due to uneven deformation between particles and the matrix, leading to particle-matrix debonding. At an external strain of 20%, microvoids coalescence causes interfacial damage and failure. The DVC method enabled the calculation of internal displacement and strain fields of the adhesive interface, revealing the deformation characteristics and strain distribution in the tensile process. The area near the liner/propellant interface is identified as the strain concentration zone in the tensile process and the primary site of damage initiation.
-
WANG Ru-yao, LI Jun-wei, WANG Xiao-dong, CAO Jun-wei, LI Qiang, WANG Ning-fei
2025,33(8):867-881, DOI: 10.11943/CJEM2025056
Abstract:To optimize the combustion performance of solid propellants and enhance the combustion stability of solid rocket motors (SRMs), an integrated combustion response model for four-component hydroxyl-terminated polybutadiene (HTPB) propellant with microcosmic heterostructure is established. The improved combustion model that considers the microstructure of four-component propellants is developed based on the heterogeneous quasi one-dimensional (HeQu1-D) framework, incorporating both the micro-scale heterogeneous structure and the unsteady heat transfer process. The model is well verified against experimental data from T-burner tests, with a maximum error of 5.34% in combustion response. Furthermore, the effects of component content distribution, particle sizes, and external environmental conditions are investigated under a working pressure of 12 MPa and excitation frequencies ranging from 250 to 2000 Hz. The results demonstrate that adjusting the particle sizes of AP and NA can significantly alter the propellant''s combustion response characteristics, where smaller AP particles combined with larger NA particles are more conducive to stable combustion. Regarding component content, increasing the relative proportion of AP helps reduce the pressure-coupled response function of propellant. When 10% of AP is replaced with RDX, the pressure-coupled response function exhibits a peak-value increase of 0.15 accompanied by a 25 Hz reduction in peak frequency. More pronounced effects are observed with HMX, where the same 10% of AP replacement leads to a greater peak value enhancement of 0.43 and a more substantial peak frequency decrease of 85 Hz. This work contributes to understanding the mechanism of combustion instability and provides guidance for efficient optimization of propellant formulations.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
-
ZHANG Xue-shen, SHEN Xiao-yin, ZHOU Hui, WANG Xue-ren, DING Li, ZHANG Dong-sheng
2025,33(8):882-891, DOI: 10.11943/CJEM2025064
Abstract:Improving the structural integrity of charge is of great significance for ensuring the working stability of solid rocket motor (SRM). Multi-angle tensile loading tests were carried out on the HTPB propellant bonded specimens. During the tensile process, binocular cameras combined with three-dimensional digital image correlation (DIC) methods were used to analyze the deformation field of the bonded specimens. According to the mesoscopic structure of the specimen, a mesoscopic cohesive zone model (CZM) was established and further subjected to numerical simulation analysis, based on three types of damage modes including particle dewetting, matrix fracture and debonding of the bonding interface. The damage evolution law, cracking mechanism and failure mode of the specimen under different tensile and shear stress states were explored. The test results show that the bonded specimen are more prone to damage under the tensile-shear mixed stress state. At the same time, the bearing capacity of the specimen decreases and a greater tensile displacement will occur with increasing the tensile angle. The area where the strain of the bonded specimen is relatively large at the critical state is the location where macroscopic cracks initiate. The numerical simulation results show that the first principal stress is the main factor affecting the generation of cracks in solid propellants, and when the value of the first principal stress is greater than 0.548 MPa, it will lead to the initiation of cracks. Furthermore, the smaller the stretching angle is, the easier the deweeting between the particle and matrix in the propellant is to occur. However, it is easier for the propellant/liner interface to de-bond and the crack propagation location is closer to this interface when the stretching angle increases.
-
LIU Fu-han, LU Hao-yang, XIA De-bin, ZHANG Jian, LIN Kai-feng, YANG Yu-Lin
2025,33(8):892-897, DOI: 10.11943/CJEM2025057
Abstract:To investigate the effect of surface current density on the combustion performance of hydrazine nitrate-based electrically controlled solid propellant (HAN-ECSP), the contact area ratio between HAN-ECSP and Cu electrode was adjusted to regulate the surface current density of propellant. Decomposition, combustion and repeated on-off combustion performances were studied using gas collection and combustion test. The results indicate that the combustion performance of propellant is improved significantly as the increase of surface current density between the cathode and propellant. However, too small effective contact area could lead to excessive local current density, potentially causing the damage of electrode. Hence, the optimal contact area ratio between anode and cathode is 1∶0.4. Compared to unchanging the contact area between electrode and propellant, the surface current density rises from 301 to 2466 A·m-2. The results of gas decomposition pressure test and combustion indicate that the total gas pressure increases by 4.8 times, gas generation rate increases by 12 times, ignition delay time of propellant decreases from 3.13 to 0.76 s and mass combustion rate increases from 0.07 to 0.22 g·s-1, when the surface current density rises 2165 A·m-2. Moreover, the propellant still maintains excellent repeated on-off combustion performance at this surface current density.
-
CHEN Ze-xin, LIU Lin-lin, YANG Yu-xin, LI Bo-biao, CHENG Cai, HU Song-qi
2025,33(8):898-906, DOI: 10.11943/CJEM2025140
Abstract:To study the combustion performance of self-extinguishing solid propellants and the pressurization-induced extinguishing mechanism, HTPB/AOT/AP self-extinguished solid propellants were prepared by using sodium dioctyl sulfosuccinate (AOT) as extinguishing agent. Experimental characterizations of burning rate, combustion wave structure, flame morphology and flame quenching burning surface topography of the propellants were carried out. Results show that AOT reduces the pressure exponent of propellant below 10 MPa from 0.37 to -1.36, and the burning rate decreases from 5.9 mm·s-1 to 2.9 mm·s-1 as the pressure approaches the critical extinguished pressure. AOT causes the propellant to self-extinguish when the pressure increases to 10 MPa, with unsustainable combustion observed at higher pressures. As the pressure approaches the critical extinction pressure, a molten layer with a thickness of 230 μm covers the burning surface of propellant, reducing the burning surface temperature from 865 K to 420 K and the gaseous phase temperature gradient from 74.9 K·μm-1 to 0.19 K·μm-1 compared with that of pressure of 2 MPa. The pressurization-induced extinguishing mechanism of self-extinguishing solid propellant is revealed. AOT causes a thicker molten layer to cover the surface of propellant, obstructs the heat feedback from the gaseous flame to the burning surface, reduces the temperature of burning surface and the reaction rate of condensed phase, and ultimately results in extinguishment due to the failure to maintain self-sustained combustion.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
-
DONG Ying-nan, JIANG Yi-fan, ZHAO Feng-qi, LI Si-heng, LIU Ding, QU Wen-gang
2025,33(8):907-927, DOI: 10.11943/CJEM2024304
Abstract:The core-shell structure can effectively suppress the large aluminum(Al) agglomerates produced by combustion of Al-matrix composites, enhance the energy release efficiency of Al powder, and improve its ignition performance and combustion energy release characteristics. Based on the characteristics of core-shell structured Al-matrix composites, an overview of the research progress was summarized. The commonly used preparation methods for core-shell structured Al-matrix composites were discussed, effects of different compositions on the combustion performance, energy release efficiency and stability of these composites were analyzed. Furthermore, the potential applications and future development directions of core-shell structured Al-matrix composites were outlined. Optimizing the preparation techniques for core-shell structures to achieve large-scale production, regulating the composition of the coating materials or constructing functional interlayers at the matrix-coating interface can effectively improve the mass and heat transfer characteristics during the combustion process of Al-matrix composites.
-
DENG Wen-wen, YE Bao-yun, QIU Mian-ji, ZHANG Zhi-yuan, WANG Ze-yu, AN Chong-wei, WANG Jing-yu
2025,33(8):928-939, DOI: 10.11943/CJEM2025085
Abstract:As an energetic polymer, glycidyl azide polymer (GAP) has the characteristics of high energy and good film-forming performance, and has a wide application prospect in the fields of propellants, explosives and so on. The latest research progress in aspects such as the optimization of GAP synthesis process, GAP modification technology, GAP-based energetic thermoplastic elastomers (ETPE), and GAP-based ETPE with self-healing function were reviewed. Firstly, two synthetic methods of GAP were analyzed. It was proposed that the development directions were to develop a safe, green and efficient synthetic routes of GAP and the improvement and engineering application of the existing optimized processes. Secondly, in view of the poor low-temperature mechanical properties and irregular structure of GAP, the improvement effects of copolymerization modification, interpenetrating network technology, microwave radiation technology and fluoropolymers on the mechanical properties and thermal stability of GAP were mainly reviewed. It was proposed that developing low-cost modification processes and conducting in-depth research on the long-term storage and environmental adaptability of GAP-modified materials were the directions to promote the engineering development of modified GAP. Then, the influence of GAP-based ETPE structure regulation on mechanical properties, thermal stability and glass transition temperature was reviewed. Especially for GAP-based ETPE with self-healing function, scholars at home and abroad have achieved active repair of micro-defects by introducing dynamic covalent bonds and supramolecular interaction mechanism, significantly improving the mechanical properties and environmental adaptability of propellants and explosives.
-
LIU Ya-jun, BAO Li-jun, BAI Rui, MO Nan-fang
2025,33(8):940-959, DOI: 10.11943/CJEM2025147
Abstract:Aluminum trihydride (AlH3) is regarded as one of the ideal high-energy fuels in the fields of composite solid propellants and explosives due to its high hydrogen content, high combustion calorific value, and low molecular weight of combustion gas. The research progress on the preparation methods, stabilization modification and application of AlH3 was systematically reviewed. The advantages and disadvantages of different preparation methods from different dimensions such as crystal form control, particle size regulation and purity optimization were deeply compared and analyzed. The latest research trends in AlH3 stabilization technology and the current research status of solid propellants containing AlH3 in terms of system compatibility and combustion performance were summarized. The challenges faced by the future engineering application of AlH3 and looks forward to the research directions that need to be urgently carried out for AlH3 as a high-energy fuel are as follows: Focusing on high-throughput screening of synthesis parameters in liquid-phase synthesis routes, precise control of heat transfer in multi-phase systems during crystal transformation, and studying on the safety control during the synthesis process to achieve safe large-scale preparation of high-quality AlH3; Conducting multi-scale modeling and performance prediction of AlH3 stabilization to promote the transformation of stabilization work from “trial and error based on experience” to “intelligent design”; Multi-scale analysis of the wet-heat stability of AlH3 and its compatibility with propellant components, conducting the design and screening of propellant material systems that are compatible with AlH3, and relying on material system innovation to solve the root problems.
Vol, 33, No.8, 2025 Innovation and Application of High-Performance Propellants
>Editorial
>Energetic Express
>Perspective
>Propulsion and Projection
>Reviews
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Propellant
2021-2023 Collection
-
-
Gun Propellant
2021-2023 Collection
-
Safety and damage study
2021-2023 Collection
-
Initiator and Pyrotechnics
2021-2023 Collection
-
Preparation and Property
2021-2023 Collection
-
Crystal and microscopic analysis
2020-2022 发表
-
Chemical Propellant
2021-2022 Collection
-
-
-
-
Eco-friendly technology
2021-2022 Collection
-
Initiating explosive device technology
2021-2022 Collection
-
Damage and ignition
2021-2022 Collection
-
Thermal decomposition,safety performance and evaluation
2021-2022 Collection
-
Preparation and performance—Characterization of molding materials
2021-2022 Collection
-
Preparation and performance—Characterization of synthesis
2021-2022 Collection
-
Preparation and performance—Study on synthesis and performance
2021-2022 Collection
-
Explosion and damage
2021-2022 Collection
-
Detonation physics of energetic materials
2021-2022 Collection
-
High efficiency destruction technology
2021-2022 Collection
-
Propulsion and projection—Propulsion Materials structure and activity relationship
2021-2022 Collection
-
Propulsion and projection—Preparation and performance about propulsion materials
2021-2022 Collection
-
Calculation and simulation—Material structure and response
2021-2022 Collection
-
Calculation and simulation—Structural evolution of materials
2021-2022 Collection
-
Calculation and simulation—Material performance prediction
2021-2022 Collection
-
-
-
-
-
-
-
-
-
-
-
-
Damage and Ignition
2020
-
Detonation Physics
2020
-
 Special Issue
Special Issue Virtual Special Issue
Virtual Special IssueCJCR